- Review
- Open access
- Published:
Polymeric nanomedicines for the treatment of hepatic diseases
Journal of Nanobiotechnology volume 20, Article number: 488 (2022)
Abstract
The liver is an important organ in the human body and performs many functions, such as digestion, detoxification, metabolism, immune responses, and vitamin and mineral storage. Therefore, disorders of liver functions triggered by various hepatic diseases, including hepatitis B virus infection, nonalcoholic steatohepatitis, hepatic fibrosis, hepatocellular carcinoma, and transplant rejection, significantly threaten human health worldwide. Polymer-based nanomedicines, which can be easily engineered with ideal physicochemical characteristics and functions, have considerable merits, including contributions to improved therapeutic outcomes and reduced adverse effects of drugs, in the treatment of hepatic diseases compared to traditional therapeutic agents. This review describes liver anatomy and function, and liver targeting strategies, hepatic disease treatment applications and intrahepatic fates of polymeric nanomedicines. The challenges and outlooks of hepatic disease treatment with polymeric nanomedicines are also discussed.
Introduction
The liver is the largest solid organ in the human body and performs a wide range of functions, including protein synthesis, metabolism, immune responses, endocrine-based regulation, biotransformation of nutrients, and detoxification. Therefore, disordered liver functions induced by various hepatic diseases, including viral hepatitis, fatty liver, hepatic fibrosis, hepatic cirrhosis, and hepatocellular carcinoma (HCC), significantly threaten human health, and result in nearly 2 million deaths per year around the world [1].
Various therapies have been applied in the clinical treatment of liver diseases, such as surgical resection, interventional therapy, chemotherapy, radiotherapy, antiviral therapy, immunotherapy, and liver transplantation (LT) [2, 3]. Although great achievements have be obtained in the clinic, some challenges limit the successful applications of current hepatic disease therapies. For example, incomplete resection may induce tumour recurrence; conventional pharmacotherapy may generate drug resistance, and result in severe systemic toxicity and limited therapeutic efficacy due to their lack of targeting ability; and the patients need to take immunosuppressant drugs for the remainder of their lives to avoid transplant rejection after LT, which may induce severe side effects and reduce their living quality [4, 5]. Especially, owing to the various and complex etiologies of nonalcoholic steatohepatitis and hepatic fibrosis, no standard medication is available for their clinical treatment despite the proved effectiveness of some therapeutic agents in clinical trials [6, 7]. Recently, nanomedicines have been reported to have the capacity to improve the therapeutic outcomes and reduce the side effects of medications (e.g., natural extracts, chemotherapeutic drugs and nucleic acid-based drugs) by facilitating liver-specific drug delivery, thus offering new paradigm to address the aforementioned challenges.
Significant progress has been made in the field of nanomedicine in terms of disease diagnosis and treatment in the past half century [8, 9]. Since Gregoriadis and coworkers developed the first liposomes in 1974 [10], various nanodrug delivery systems have been developed. These carriers typically include biomacromolecules (e.g., proteins), inorganic materials, viral capsids, polymers, and lipids. The excellent physicochemical properties of nanoparticles (NPs) provide them with superior advantages, including improved drug pharmacokinetics, cell and tissue gap penetration ability, enhanced drug accumulation at diseased sites by passive or active targeting strategies, controlled drug release, reversing multidrug resistance, enabling high contrast imaging, and reductions in side effects [11, 12]. The commercialization of nanodrugs (e.g., Caelyx/Doxil, and Smarticles) has led to great successes in tumour therapy and in infectious disease therapy (e.g., Ambisome®) [12, 13]. Furthermore, nanomedicines based on various materials have been developed to treat hepatic diseases [12, 14]. Among them, lipid- and polymer-based nanomedicines have been used most frequently due to their well-established synthesis and characterization methods, high biocompatibility, and biodegradability [11]. However, the further development of lipid nanomedicines is restricted to some extent by their limited stability (e.g., the fragile nature of lipid bilayer of liposomes) and difficulties in functionalization of lipids [15]. Polymeric nanomedicines, in contrast, can be easily modified to attain ideal physicochemical characteristics and functions, such as high stability, stimuli-responsive properties, and targeting ability, and therefore have been widely developed for the treatment of various hepatic diseases, such as hepatitis B virus (HBV) infection, nonalcoholic steatohepatitis (NASH), liver fibrosis, HCC, and host-versus-graft disease (HVGD) (Fig. 1) [11].
Polymer-based nanomedicines can be roughly classified into several categories: polymer conjugates, dendrimers, nanogels, polymeric micelles, polymeric nanocapsules, and lipid-polymer hybrid NPs (Fig. 2) [16]. Some polymer NPs have been approved for patient use. For example, Zoladex, a poly(lactic-co-glycolic acid) (PLGA) copolymer carrying goserelin acetate for the treatment of breast cancer and prostate cancer, was approved in 1998. Trelstar Depot, a triptorelin pamoate microparticle with a PLGA carrier for use in the treatment of advanced prostate cancer, was approved in 2000. A paclitaxel polymeric micelle (Genexol-PM) used for treating malignant tumours was approved in Korea in 2007 [17, 18]. Although several polymeric nanomedicines for the treatment of HCC and NAFLD underwent clinical trials, none of them have yet been approved (Table 1). Therefore, great efforts continue to be needed to promote the development of polymer-based nanomedicines for the treatment of hepatic diseases. This review describes liver anatomy and function, and liver targeting strategies, hepatic disease treatment applications, and the intrahepatic fates of polymer-based nanomedicines. The challenges and outlooks for effective therapy of hepatic diseases using polymeric nanomedicines are also discussed.
Hepatic anatomy and function
The liver is under the ribcage on the right-hand side of the abdomen (Fig. 3a), and accounts for approximately 2–3% of the body weight [22]. The blood of the liver is supplied mainly by the hepatic artery which contributes 25% of the liver blood supply but 75% of the oxygen, and by the portal vein which contributes 75% of the supplied blood (Fig. 3a). Typically, the liver consists of parenchymal cells and nonparenchymal cell (NPCs). The parenchymal cells include hepatocytes, which account for 70–80% of the cells in the liver and are the principal cell type in liver [23]. The NPCs mainly include liver sinusoidal endothelial cells (LSECs), Kupffer cells (KCs), and hepatic stellate cells (HSCs) [24]. LSECs form the lining of the hepatic sinusoid and have fenestra with pore size of approximately 100–200 nm [25, 26]. KCs account for 80–90% of the total number of macrophages in the body, and are intrinsic parts of the reticuloendothelial system (RES). HSCs make up nearly one-third of the NPCs [27, 28], reside in the space of Disse, and directly contact LESCs, hepatocytes, and other HSCs [29]. The detailed functions of parenchymal cells and NPCs are shown in Table 2, together with various hepatic diseases associated with different cell types. Furthermore, the diseased cells overexpress some specific receptors that can be used for targeted therapy of according hepatic diseases (Fig. 4).
The functional and structural unit of the liver is a hepatic lobule, which measures approximately 2.0 × 0.7 mm and is composed of hepatocyte plates organized in a solid hexagonal shape surrounding the central vein. It is separated by the anastomosing system of sinusoids that perfuse cells with a mixture of portal and arterial blood. A portal triad containing the hepatic artery, the portal vein, and the bile ducts is arranged around each vertex of the hepatocyte-formed hexagon (Fig. 3b) [30,31,32]. Moreover, the hepatic sinusoid has a diameter of approximately 5 to 10 μm, and could facilitate the exchange of substances between the blood and the perisinusoidal space of Disse (Fig. 3c) [30].
Strategies for hepatic-targeted drug delivery
To treat hepatic diseases precisely, liver-targeted drug delivery systems have been developed and have made considerable progress in this area. In summary, nanomedicines can passively or actively target disease sites. The passive accumulation of NPs in the liver depends on the properties of the NPs, structure of hepatic lobules, pathophysiological features of liver diseases, and mode of drug administration. Passive accumulation of nanomedicine in target tissues increases local drug concentration and uptake by diseased cells, thereby reducing drug distribution to healthy organs. Active targeting, however, depends on the cell-specific ligands decorating the surface of NPs, because these ligands can recognize and bind to the specific receptors on certain types of cells, thereby preventing unspecific toxicity on normal liver cells. In fact, these two targeting strategies are often performed concurrently; NPs first enter the liver by passive diffusion and are subsequently internalized through receptor-mediated endocytosis (Fig. 5).
Passive targeting
Following intravenous administration, NPs that carry a significant surface charge may be rapidly covered with a layer of proteins and may elicit immune responses, leading to their uptake by KCs in sinusoids [33, 34]. Research has shown that NPs with negatively charged surfaces are taken up at an increased rate by the RES in the liver, while hepatocytes are prone to internalize NPs with cationic surface charges [12, 34]. In addition, compared to hydrophilic NPs, hydrophobic NPs are more rapidly eliminated from blood by the RES [34]. Therefore, NPs are often polyethylene glycol (PEG)ylated to prolong their blood circulation, reduce protein binding, and minimize their uptake by the RES.
In addition, NP size plays a crucial role in the targeted therapy of hepatic diseases. NPs with a diameter smaller than 200 nm and without being taken up by KCs can enter the space of Disse through slightly larger-than-average fenestrations, through which they diffuse to various liver cells [11]. The smaller the size of the NPs is, the easier they pass through the fenestrations deep into the space of Disse and are internalized by hepatocytes [35]. Surprisingly, studies have shown that nanocarriers as large as 400 nm can enter the space of Disse and pass through the fenestrations via a mechanism of forced extrusion [36]. The shape of designed NPs can also affect NP distribution. Rod-shaped and worm-like NPs with very high aspect ratios are not taken up as rapidly by KCs as spherical shaped NPs [37, 38].
Passive accumulation of nanomedicine in some solid tumours can be realized through the enhanced permeability and retention (EPR) effect, which is caused mainly by the immature differentiation of tumour vasculature and impaired lymphatic drainage [39,40,41,42]. The diameter of the fenestrations is reported to vary between 400 and 600 nm in human tumours [43]. Therefore, NPs are also widely used in passive targeted therapy of HCC by EPR effect [44,45,46]. In addition, some studies have indicated that the optimal diameter of NPs used for cancer treatment is between 70 and 200 nm [47].
In addition to particle properties, the structure of hepatic lobules, the pathophysiological features of HCC, and the method and site of administration also affect liver cell uptake and intrahepatic distribution of nanomedicines. For example, some methods, including hydrodynamic injections, and intra-arterial, intratumoral and intrabiliary infusions, have been employed to facilitate the accurate diagnosis and treatment of liver diseases [11, 12].
Active targeting
Active targeting, also known as ligand–receptor-mediated targeting, involves the use of peripherally conjugated targeting moieties for the specific uptake of nanomedicines by target cells [42, 48]. Targeting moieties, such as antibodies, proteins, peptides, aptamers, carbohydrates, and vitamins (e.g., folate and retinol) (Table 3), can target surface molecules or receptors overexpressed on disease cells [48, 49]. As mentioned above, various receptors are expressed on the surface of liver cells and can be used to develop liver-targeted nanomedicines (Table 3).
The concept of targeted NPs has been implemented for more than 40 years, but only a minority of these NPs have entered into clinical trials, and none are currently approved for clinical application [17]. Many factors influence the targeting ability of NPs, including (i) the administration route of the NPs, such as the oral, intramuscular, intravenous, or intratumoral routes, and (ii) the physicochemical properties of the NPs, such as the types and density of the ligands, as well as the size, materials, shape, charge, and surface hydrophobicity of the NPs [42]. Therefore, improvement of the active targeting ability of nanomedicines is needed to promote their clinical translation.
Polymer-based nanomedicines for liver disease treatment
Liver diseases are related to pathological changes in the structure and function of liver cells, which are usually induced by endogenous or exogenous pathogenic factors [93,94,95,96]. Common liver diseases associated with different cell types are summarized in Table 2. Based on the current understanding of pathophysiological mechanisms of hepatic diseases at the cellular level and the understanding of the macroscopic and microscopic anatomy of the liver, researchers have designed many drug delivery systems in recent years, and they have shown excellent application possibilities. In the following section, we review the progress in the development of various polymer-based nanocarriers that show promises for the specific delivery of therapeutic agents to treat HBV infection, NASH, hepatic fibrosis, HCC, and HVGD after LT (Table 4).
Hepatitis
Hepatitis is characterized by structural and functional impairment of liver cells and the infiltration of inflammatory cells and inflammatory factors. Long-term alcoholism, exposure to toxic agents, and viral/bacterial infection can eventually lead to liver damage. HBV infection and NASH are the two most common chronic hepatic diseases, and no efficient therapy is available for them thus far. In recent years, many attempts have been made to improve the treatment effect of hepatitis.
HBV infection
HBV is a hepatotropic DNA virus that can cause a lifelong chronic infection. HBV replication in hepatocytes can cause liver fibrosis, cirrhosis, and HCC. Although HBV surface-antigen vaccines are effective in preventing HBV infection, it is difficult to eliminate HBV from infected cells. Various antiviral agents, including interferons, nucleic acids, and small molecules, have been used to control HBV infections. However, these therapeutics lead to dose-limiting side effects and may induce the development of drug resistance [131]. Various polymeric nanocarriers have been developed to deliver classical antiviral drugs (vaccines, nucleic acids, and small molecules) to hepatocytes for HBV infection treatment, which effectively reduce the side effects and enhance the therapeutic outcomes of drugs [8, 11].
-
(i)
Vaccine delivery: The HBV vaccine refers to inactivated HBsAg, which is the mainstay of hepatitis B prevention. However, the poor absorbability, low immunogenicity, and poor patient compliance in receiving the traditional inoculation inhibit the further development of vaccines. Hence, some alternative routes of administration have been developed to eliminate these difficulties. The nasal mucosa contains a rich capillary plexus, through which the vaccines can enter the blood quickly; therefore, Subbiah et al. formulated a N,N,N-trimethyl chitosan (CS) nanocarrier to deliver HBsAg (N-TMC NPs) through the nasal route. The NPs increased the drug-loading rate and prolonged antigen release in vitro, and an immunological study revealed that the adjuvant efficiency of these NPs for the antigen was highly stable and better than the standard efficiency in vivo [97]. Similarly, nasal vaccination has also been performed with nanovaccines consisting of poly-ε-caprolactone (PCL)/CS and HBsAg based on the stability of PCL in the blood circulation and the mucoadhesive and immunostimulatory properties of CS. The combined delivery of PCL and CS produced a synergistic effect in the efficient generation of an immune response [132]. Furthermore, Dewangan et al. designed a variety of NPs based on PLGA to deliver HBsAg and utilized a central composite design for formulation optimization. The results showed that the intramuscular delivery of the nanovaccine resulted in stronger humoral and cellular responses [133, 134]. Zhu et al. loaded HBsAg onto mannose-modified PLGA NPs, which slowly released HBsAg and enhanced antigen presentation to lymphocytes, resulting in long-term immunity. The study showed that NP uptake by bone marrow-derived dendritic cells (BMDCs) and RAW 264.7 cells was significantly increased. In addition, subcutaneous delivery of NPs maintained humoral immunity and enhanced cellular immune responses in animal tests [77]. Moreover, Wang et al. prepared a HBsAg nanogel (Ng) using CS and poly-γ-glutamic acid (γ-PGA). The results indicated that HBsAg Ng not only boosted the immune system but also promoted the proliferation of memory T cells. Moreover, the positively charged Ng (+) was more stable and provided longer protection against HBV than the negatively charged Ng (−), making it desirable as a HBsAg vaccine carrier [101].
-
(ii)
Nucleic acid delivery: In recent years, gene therapy has been widely applied to the treatment of HBV infections. CS and its derivatives have been widely used to fabricate gene delivery vectors [135]. For example, Zeng et al. fabricated NPs using only PLGA, and the NPs initially showed low capacity for plasmid DNA (pDNA) encapsulation; however, by combining PLGA with CS as a carrier, the retention of anionic pDNA, encapsulation efficiency and drug loading capacity were all increased. CS-modified PLGA NPs showed a positive zeta potential and were effectively taken up by the cells, which was conducive to antiretroviral therapy in vivo [98]. In addition, cell-penetrating peptides (CPPs) possess a significant capacity for membrane transduction and can deliver various bioactive molecules into cells. Therefore, CPPs combined with CS may be used as an ideal nonviral vector to deliver peptide nucleic acids (PNAs) or DNA vaccines to treat HBV [136]. DrzBC and DrzBS (10–23 DNAzymes) effectively inhibited the expression of HBV e- and s-genes, respectively, and greatly reduced viral load. Therefore, Miao et al. designed a CS-based glycolipid-like nanocarrier (CS oligosaccharide-SS-octadecylamine, CSSO) with redox-responsive and endosomal escape properties. This carrier bound with DrzBC and DrzBS DNA via electrostatic interactions to form CSSO/DrzBC and CSSO/DrzBS complexes. Studies showed that CSSO/DNA powerfully inhibited HBV replication [100].
-
(iii)
Synthetic small-molecule delivery: Bay41-4109 is an effective inhibitor of HBV replication. Jiang and coworkers prepared Bay41-4109-loaded CS NPs. The NPs significantly improved the bioavailability of Bay41-4109 and provided an effective method for the treatment of HBV [99]. In addition, PLGA microspheres with the capacity of sustained release were prepared and loaded with adefovir and entecavir. The microspheres reduced the medication dose and frequency in patients with chronic hepatitis B [137,138,139]. Recently, Hamdi et al. synthesized lipid polymer hybrid (LPH) NPs with the merits of polymers and liposomes to deliver entecavir. The physicochemical properties of the vitamin E-modified LPH NPs were favourable and confirmed by related tests. The NPs targeted macrophages and showed enhanced the retention in J774 macrophage cells, thereby reducing viral replication in these macrophages [140].
Nonalcoholic steatohepatitis
NASH is an inflammatory subtype of nonalcoholic fatty liver disease (NAFLD), to which alcohol consumption is not a contributor [141,142,143]. The development of NASH involves many molecular pathways, and various pathogenic factors lead to highly heterogeneous diseases and clinical manifestations [144]. A widely accepted explanation involves the inability of the liver to catabolize an overabundance of carbohydrates and fatty acids, which leads to an increase of toxic lipid species in hepatocytes [6, 142, 145,146,147]. These primary metabolic energy substrates induce hepatocellular stress, injury, and death, leading to fibrogenesis and genomic instability, and increasing the risk of cirrhosis and HCC [142].
In recent years, with increasing awareness of the pathogenesis of NAFLD, great progress has been made in the research and development of various drugs, but significant challenges remain unresolved, and no drugs have been approved for clinical use. Fortunately, several medications have been proved to be effective at reducing steatohepatitis in clinical trials, including weight loss medications, insulin sensitizers, vitamin E, cholesterol-reducing medications, cytoprotective agents, and obeticholic acid [143]. However, the efficacy and safety of these drugs require further evaluation. To improve the drug treatment effect on NAFLD, novel polymeric nanomedicines have been recently developed.
-
(i)
Natural extract delivery: Resveratrol (Res), a phytoalexin extracted from grapes and other food product, can regulate blood lipid and blood glucose homeostasis and relieve metabolic disorders. However, the application of Res is limited due to its poor bioavailability and stability. Therefore, researchers improved its physicochemical characteristics by loading it into a galactose (Gal)-modified polymer carrier. Results showed that the Res-loaded nanomedicines effectively prevented NAFLD progression compared with free Res [104, 106]. Both CS, a natural cationic aminopolysaccharide, and silymarin, a main component in milk thistle extracts, have the ability to lower lipids. Therefore, Liang et al. synthesized CS-modified, silymarin-loaded LPH NPs with a shell-core structure consisting of a polymer core and a phospholipid shell to enhance the oral bioavailability and improve the lipid-lowering efficacy of silymarin in NAFLD treatment [148].
-
(ii)
Synthetic small-molecule delivery: De novo lipogenesis (DNL) is relevant to sterol regulatory element-binding protein (SREBP)-1c and is significantly increased in NAFLD patients. Therefore, DNL may be a potential therapeutic target. Zhao et al. prepared mPEG-PLGA loaded with rapamycin (RAPA) to treat NAFLD, which significantly decreased the lipid content, attenuated hepatic steatosis, and repaired liver injury in mice [102]. Cao et al. studied the therapeutic efficacy of biodegradable polyurethane NPs loaded with a PPARα agonist fenofibrate (FNB) on NAFLD. They demonstrated that the NPs dramatically reduced triglyceride levels both in vitro and in vivo, and increased the plasma FNB concentration of mice [109]. Nifedipine (NFD) suppressed the high-fat diet-induced accumulation of p62 and ubiquitinated protein inclusions by restoring cytosolic calcium homeostasis and inducing autophagy and lysosomal degradation. Recently, NFD-NPs that alleviated obesity-related metabolic dysfunction were developed using PLGA as the carrier. NFD-NPs increased the concentration of NFD and restored lipid metabolism associated with NAFLD by enhancing autophagy-based clearance in the liver, thereby increasing the sensitivity of cells to insulin and attenuating glucose tolerance (Fig. 6) [105]. In addition, R406 inhibits splenic tyrosine kinase pathway activation, which is related to the pathogenesis of NASH and alcoholic hepatitis. Kurniawan et al. synthesized R406-loaded PLGA NPs to improve the pharmacokinetics of R406, which ameliorated fibrosis, inflammation, and steatosis in mice, restoring liver function and reducing plasma lipid levels [107].
Fig. 6 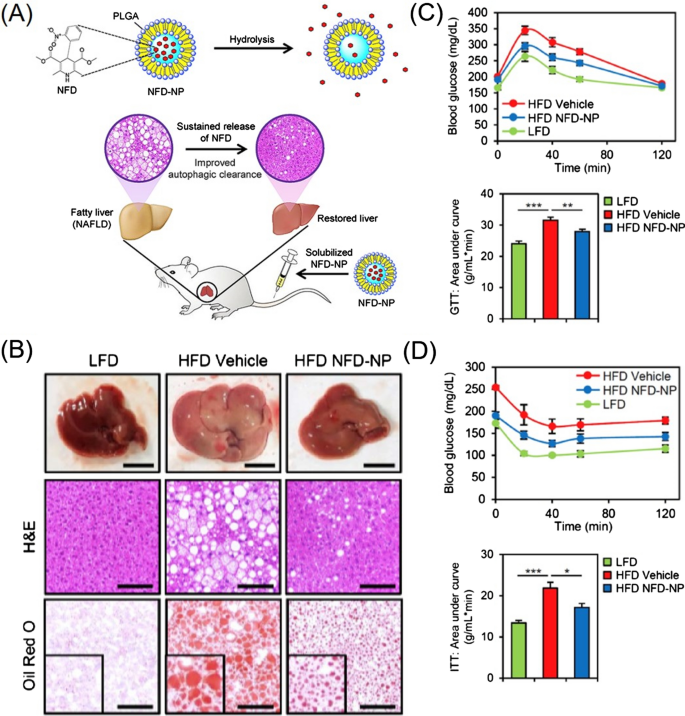
(Reprinted with permission from Ref. [105], Copyright 2019, Elsevier Ltd)
A Schematic illustration of Nifedipine nanoparticles (NFD-NPs) for preventing NAFLD. B NFD-NPs alleviate high-fat diet (HFD)-induced obesity and hepatic steatosis. C and D NFD-NPs improve insulin sensitivity and glucose tolerance.
-
(iii)
Nucleic acid delivery: IL-22, an effective agent for alleviating NAFLD, might induce severe adverse effects at high doses. Therefore, biguanide and CS were used to synthesize a novel polymetformin carrier that can be combined with penetratin and DSPE-PEG2000 to form stable nanocomplexes with the IL-22 gene. NPs containing IL-22 and metformin exhibited dual therapeutic effects on NAFLD, remarkably alleviating hepatic steatosis, restoring insulin sensitivity, and attenuating metabolic syndrome in animal tests [103]. Furthermore, a miR-146b mimic exhibited potent anti-inflammatory activity in NASH; therefore, the miR-146b mimic was targeted to hepatocytes in NAFLD mice with lactosylated poly(N,N-dimethylaminoethyl methacrylate) (Lac-PDMAEMA) as the carrier. These NPs effectively suppressed the expression of PPARγ and decreased the levels of TNF-α and IL-6 mRNA, ultimately alleviating hepatic steatosis [108].
Liver fibrosis
Liver fibrosis is the primary cause of mortality in patients with chronic hepatic disease [149]. The common causes of fibrosis include chronic hepatitis B or C infections, alcohol abuse, NAFLD, autoimmune liver disease, and hereditary diseases [150]. The mechanisms of liver fibrogenesis include: (i) reactive oxygen species (ROS) and other oxidative stress-related mediators induce a chronic inflammatory response to activated HSCs (aHSCs) in the liver [151, 152]; (ii) extracellular vesicles released by injured and/or apoptotic hepatocytes affect almost all cell populations, inducing/sustaining inflammation, fibrosis and angiogenesis [153]; and (iii) excess ECM induces remarkable changes in the quality and morphological distribution of ECM components, especially type I and III collagen, due to imbalanced synthesis and degradation of collagen fibres and increased expression of inhibitors of metalloproteinases (TIMPs) [154]. HSCs are activated and transformed into hepatic myofibroblasts (HMFs) after hepatic injury [153]. aHSCs play vital roles in the synthesis and secretion of excessive ECM in response to damaged hepatocytes, LSECs and KCs. Therefore, aHSCs are hallmarks of hepatic fibrosis and are known as major targets for the treatment of hepatic fibrosis [155].
Hepatic fibrosis can be attenuated either by delaying the progression of fibrosis and/or by promoting the resolution of fibrosis. The primary treatment strategy for hepatic fibrosis is withdrawal of all pathogenic microorganisms and chemicals that continuously damage the liver parenchyma [153]. Many drugs, plant and animal extracts, and monoclonal antibodies (mAbs) are being developed for treating hepatic fibrosis [12, 14, 156, 157], including IFN-γ, pirfenidone, vitamin E, colchicine, anti-CCL24 mAbs, anti-PDGF-B mAbs, polydatin, renin-angiotensin system inhibitors, silymarin, and nucleic acids [7, 158]. Nevertheless, none of these candidates are effective in stopping or reversing hepatic fibrosis in clinical trials due to insufficient drug delivery to the fibrotic liver which has changed macrostructure and microenvironment. Therefore, no agents have been approved as antifibrotic drugs [159]. To overcome the barriers to drug development, the application of nanotechnology has attracted increasing attention.
-
(i)
Natural extract delivery: Natural extracts are considered sources of novel bioactive substances due to their excellent antifibrotic, antihepatotoxic and antioxidant properties [157, 160]. As hyaluronic acid (HA) can specifically bind to CD44 receptors, which are highly expressed on the surface of aHSCs, Li et al. synthesized a silibinin-loaded HA (SLB-HA) micelle to treat hepatic fibrosis. The micelles showed superior targeting to fibrotic liver and specifically bound to and killed aHSCs, leading to an excellent anti-hepatic fibrosis effect [161]. In addition, Lin et al. designed a polydatin-loaded micelle (PD-MC) based on a ROS and pH dual-sensitive block polymer PEG-poly(2-((((4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzyl)oxy) carbonyl) oxy)ethyl methacrylate co 2-(diisopropyl amino)ethyl methacrylate) (P(PBEM-co-DPA). The micelle shows excellent liver-targeted drug release in response to high ROS levels and acidic environments. Moreover, the PEG-p(PBEM-co-DPA) micelle can deplete ROS at the pathological site to exert anti-inflammatory effects. Results have shown that PD-MC can dramatically inhibit inflammatory reactions and oxidative stress, decrease hepatocyte apoptosis, and prevent the activation of macrophages and HSCs [162]. In addition to plant extracts, animal extracts have also been delivered to aHSCs as antifibrotic drugs. For instance, astaxanthin is a keto-carotenoid in the terpene class of chemical compounds, and it is abundant in marine animals, such as salmon and shrimp. Astaxanthin has shown antioxidant and anti-inflammatory activities. Hu et al. prepared biopolymer-based NPs using a stearic acid-CS conjugate (SA-CS) and sodium caseinate to load astaxanthin. These NPs dramatically enhanced LX-2 cellular bioactivity by reducing TGFβ1-induced fibrogenic gene expression levels, as well as α-SMA and COL1A1 protein levels, compared to free astaxanthin [163].
-
(ii)
Synthetic small-molecule delivery: Tyrosine kinase inhibitors (TKIs) (e.g., sorafenib and nilotinib) exhibit powerful antifibrotic activity. Lin et al. developed a mixture of PEG-PLGA and PLGA to transport sorafenib, which increased the uptake by fibrotic liver cells and decreased α-SMA levels and collagen production. Furthermore, these NPs dramatically reduced the size of abnormal blood vessels and reduced microvascular density, restoring the normal function of the vessels in the fibrotic liver [164]. To increase the permeability of nanoformulations and accurately target aHSCs, Fan et al. developed collagenase I and retinol co-modified polymeric micelles that have nanodrill-like and HSC-targeting properties, based on PLGA-PEG-Mal, to load nilotinib for liver fibrosis therapy. This micelle efficiently degraded pericellular collagen I and exhibited increased uptake by HSCs. Moreover, these micelles showed excellent accumulation in the fibrotic liver and accurate targeting to aHSCs, showing optimal antifibrotic activity (Fig. 7) [165].
Fig. 7 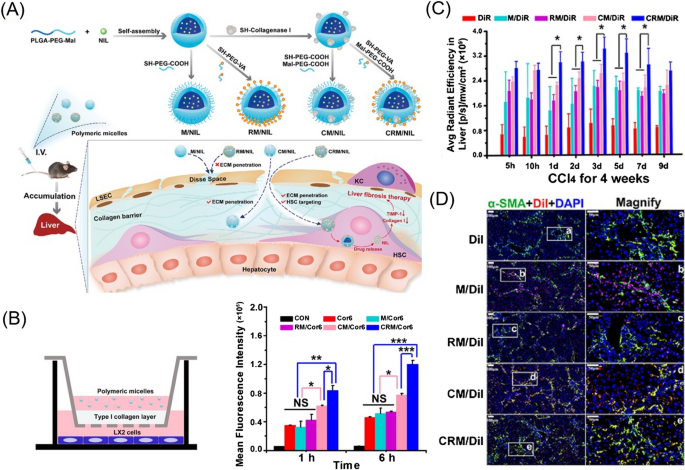
(Reprinted with permission from Ref. [165], Copyright 2019, Elsevier Ltd)
A Schematic illustration showing the preparation of polymeric micelles and their proposed destinations in vivo. B Cellular uptake of polymeric micelles through anti-collagen I barrier activity in vitro. C Fluorescence intensity in the liver of normal mice and mice treated with CCl4 for 4 weeks, expressed as average radiant efficiency units. D Colocalization of DiI and DiI-labelled polymeric micelles with activated HSCs in the liver of fibrotic mice.
-
(iii)
Nucleic acid delivery: Novel nucleic acid-based antifibrotic agents show specificity and efficacy, but their use in the treatment of fibrosis becomes a heavy burden for patients, since repetitive and long session of parenteral administration are necessary, as most nucleic acids are rapidly identified and degraded by nucleases in the bloodstream. Therefore, development of satisfactory biocompatible and biodegradable carriers to deliver nucleic acids is urgently needed. A pH-sensitive vitamin A (VA)-conjugated copolymer VA-PEG-polyethyleneimine-poly (N-(N′,N′-diisopropylaminoethyl)-co-benzylamino) aspartamide (T-PBP) was developed and assembled into superparamagnetic iron oxide-decorated cationic micelles for miRNA-29b and miRNA-122 delivery [166]. The T-PBP micelle efficiently delivered the microRNAs (miRNAs) in a manner that allowed magnetic resonance imaging (MRI). A synergistic antifibrotic effect was realized through suppressing the expression of fibrosis-related genes, including collagen type I alpha 1, ɑ-SMA, and TIMPs. This study revealed excellent antifibrotic efficacy in terms of improved liver function and alleviated liver fibrosis in vivo. Ketal cross-linked cationic nanohydrogel particles were also fabricated to deliver siRNA, which were degraded at endosomal pH and thus released siRNA. More importantly, the particles accumulated in fibrotic tissue, facilitating the knockout of sequence-specific genes related to fibrosis [167]. Zhang et al. developed a retinol-conjugated polyetherimine (RcP) NP to selectively adsorb retinol binding protein 4 (RBP) as its corona components. The RBP-incorporated NPs could deliver antisense oligonucleotide to HSCs, effectively downregulating the expression of collagen I and subsequently alleviating fibrosis [168].
-
(iv)
Codelivery of antifibrotic agents: The synergistic therapeutic effectiveness of chemical and nucleic acid drugs has been confirmed in liver fibrosis. Qiao et al. prepared a nanomicelle with poly(lactide-co-glycolide)-polyspermine-PEG-vitamin A (PLGA-PSPE-PEG-VA) for the codelivery of silibinin and siCol1α1. The VA added to the surface of the NPs specifically bound to the retinol-binding protein receptor on aHSCs in the fibrotic liver, which led to more efficient reduction of collagen I production and significant alleviation of hepatic fibrosis [86]. In addition, Ji et al. reported that germacrone and miR-29b were coencapsulated into PEG-PLGA based NPs. The prepared NPs were decorated with cyclic RGD (cRGD) peptides. These NPs exhibited great ability to target the fibrotic liver in mice because of the cRGD modification, inducing high cytotoxicity in aHSCs and dramatically suppressing the production of type I collagen [88].
Hepatocellular carcinoma
HCC is the most common form of hepatic cancer and accounts for ~ 90% of liver cancer cases [169]. More than 90% of HCC cases are diagnosed in patients with chronic hepatic disease, mostly as a result of hepatic inflammation [170]. In the early stages of HCC, the lesion can be removed by surgical resection, LT and nonsurgical local ablation techniques. For intermediate-stage HCC, transarterial chemoembolization (TACE) has been the most widely used treatment method for the past 20 years [171]. In addition, patients with advanced disease will first receive systemic therapies with conventional chemotherapeutics, immune checkpoint inhibitors (ICIs), TKIs or mAbs [2]. Among all the anticancer agents, sorafenib and lenvatinib remain the most effective single-drug therapies [171]. Nevertheless, combination treatments, such as the combination of ICIs with TKIs or PD1/PDL1 axis inhibitors with CTLA4 inhibitors, are promising future therapeutic strategies.
However, the insufficient distribution of drugs in tumours and the multidrug resistance of tumour cells reduce the therapeutic outcomes of anticancer agents [5]. The proper carriers can enhance the therapeutic effect and decrease side effects [45, 46, 172]. Several polymeric nanocarrier systems have shown promise in the treatment of HCC in experimental studies and will be discussed based on the classification of antitumor agents including natural extracts, chemotherapeutic drugs, and nucleic acids.
-
(i)
Natural extract delivery: Natural compounds are being investigated as anticancer drugs in view of their excellent therapeutic potentials. To maximize drug efficacy, some novel intelligent delivery nanoplatforms have recently been constructed. For example, a smart core-crosslinked camptothecin (CPT) prodrug micelle was prepared based on a phenylboronic acid-modified PEG-polyglutamic acid polymer with disulfide-bonded CPT. This micelle exhibited enhanced cellular uptake, good reduction sensitivity, and significant in vitro and in vivo antitumor efficacy [173]. Ursolic acid, a pentacyclic triterpene acid derived from many plants, has many pharmacological effects, including antioxidant, anti-inflammatory, antibacterial, anticancer, and antifungal properties. Shen et al. developed an amphiphilic self-assembled nanodrug consisting of ursolic acid, lactobionic acid and low-polyamidoamine dendrimers, which increased cytotoxicity against hepatic cancer and attenuated the migration and adhesion of SMMC7721 cells by inhibiting metastasis-related protein MMP-9 expression [56]. Polyphenols are plant-derived dietary compounds that can prevent certain chronic diseases, such as cardiovascular disease, neurodegenerative diseases, and tumours. For example, curcumin was encapsulated into the core of poly-l-lysine (PLL)-based NPs for pH-sensitive controlled release. These NPs enhanced the cellular uptake of curcumin into the human hepatoma Hep3B cell line by electrostatically absorptive endocytosis and showed prolonged blood circulation time [174].
-
(ii)
Chemotherapeutic drug delivery: Chemotherapeutic drugs have various drawbacks, such as poor solubility and short half-life in the circulatory system. Multiple polymeric nanocarriers have recently been developed to solve these problems. Owing to their biocompatibility, biodegradability, and low immunogenicity, naturally occurring polysaccharides, such as CS-based NPs, have been used for transporting doxorubicin (DOX) [55, 175]. In one study, a CS-based nanoplatform was functionalized by dual ligands (lactobionic acid and glycyrrhetinic acid) to target HepG2 cells and improve intracellular drug uptake [55]. The use of all-trans retinoic acid (ATRA), a potent Pin1 inhibitor, in solid tumours has been limited because ATRA has a relatively short half-life in blood. Therefore, Yang et al. designed a novel formulation of ATRA based on poly-l-lactic acid for effective HCC therapy. The as-prepared formulation significantly enhanced the suppression capabilities of ATRA on HCC cell growth, increasing the half maximal inhibitory dose (IC50) by more than threefold, compared to that of free ATRA [176]. In addition, sorafenib-loaded polymeric NPs were prepared by self-assembly of TPGS-b-poly(caprolactone) (TPGS-b-PCL), Pluronic P123 and SFB, followed by conjugation with an anti-GPC3 antibody for targeted treatment of liver cancer. These antibody-conjugated NPs increased cellular uptake by HepG2 cells and enhanced cytotoxicity compared with untargeted NPs [177].
Intelligently controlled release of anticancer agents based on responses to external stimuli or tumour environment stimuli has been widely leveraged. Wang et al. constructed ultrasound-responsive drug delivery NPs based on poly(lactobion-amidoethyl methacrylate) to achieve on-demand ultrasound-induced DOX release [178]. Yan et al. developed a smart acid-responsive micelle based on glycyrrhetinic acid-modified CS-polyethyleneimine-4-hydrazinobenzoic acid-DOX for the targeted delivery and pH-responsive release of DOX, which resulted in efficient targeted killing of HepG2 cells [179]. Similarly, redox-responsive theranostic NPs based on poly-(N-ε-carbobenzyloxy-l-lysine) (PZLL)-grafted HA copolymers were designed for the targeted delivery of superparamagnetic iron oxide (SPIO) and DOX for use in HCC diagnostics and therapy; this method made it easy to obtain real-time information on the biodistribution of DOX to quantitatively measure intracellular drug uptake and evaluate the treatment effect on cancer [68].
-
(iii)
Nucleic acid delivery: MiRNA-122 (miR-122) can inhibit hepatocarcinogenesis and progression, is prone to degradation in the blood and cannot effectively accumulate at a tumour site. Therefore, Guo et al. developed an ultrasound-triggered phase-transitioning and size-changing cationic nanodroplet, perfluoropentane/C9F17-PAsp(DET)/miR-122/poly(glutamic acid)-g-MeO-PEG ternary nanodroplets, to deliver miR-122. The nanodroplets combined with ultrasound radiation significantly inhibited the growth, migration, and invasion of HCC cells [180]. In addition, short GC-rich DNA (GCD) interfered with the polymerization of microtubules and induced cell apoptosis and cell cycle arrest, resulting in effective tumour cell killing. Yang and coworkers synthesized phenylboronic acid-modified polyamidoamine and used it to deliver GCD, leading to effective suppression of cell migration and invasion [181]. Similarly, a novel copolymer composed of low-molecular-weight polyethylenimine (PEI) cross-linked with myoinositol and conjugated with a galactose-grafted PEG chain was synthesized to deliver a plasmid encoding the IL15 gene. These polyplexes showed improved transfection efficiency, effectively suppressing tumour growth and prolonging the survival time of tumour-bearing mice after intraperitoneal injection, and are, therefore, a promising gene delivery system for immunogene therapy in HCC [182].
-
(iv)
Codelivery of antitumor agents: Sorafenib (SFB) has been the only available standard of care for advanced HCC for a decade. However, it is prone to induce resistance in liver cancer cells, as mentioned above. Codelivery of a small-molecule inhibitor of the PI3K/mTOR pathway (BEZ235) and SFB increased SFB therapeutic effectiveness against HCC and, notably, enhanced the treatment effectiveness of SFB-resistant HCC [183]. The strategy for efficient codelivery of nucleic acids and drugs is profoundly and negatively affected by premature drug leakage in circulation and serious off-target-associated side effects. Ning et al. reported the codelivery of hepatic-specific miR-122 and the antitumor agent 5-fluorouracil (5-Fu) by exploiting a macromolecular prodrug pathway. The complexes showed increased stability, efficiently inhibited the growth of tumour cells, further induced the apoptosis of HCC cells, and downregulated the expression of ADAM17 and Bcl-2 (Fig. 8) [184]. P-glycoprotein (P-gp) promotes drug efflux, while decreasing pro-survival Bcl-2 expression is an important goal in the treatment of liver cancer. To this end, Cheng et al. employed an amphiphilic poly[(R)-3-hydroxybutyrate] (PHB)-b-poly(2-(dimethylamino)ethyl methacrylate) (PDMAEMA) cationic polyester to deliver paclitaxel (PTX) and the Bcl-2 convertor Nur77/ΔDBD gene to effectively suppress drug-resistant HepG2/STC2 and SMCC7721/STC2 cell proliferation, partially erode P-gp-induced PTX efflux and activate the apoptotic function of the pro-survival protein Bcl-2 [185].
Fig. 8 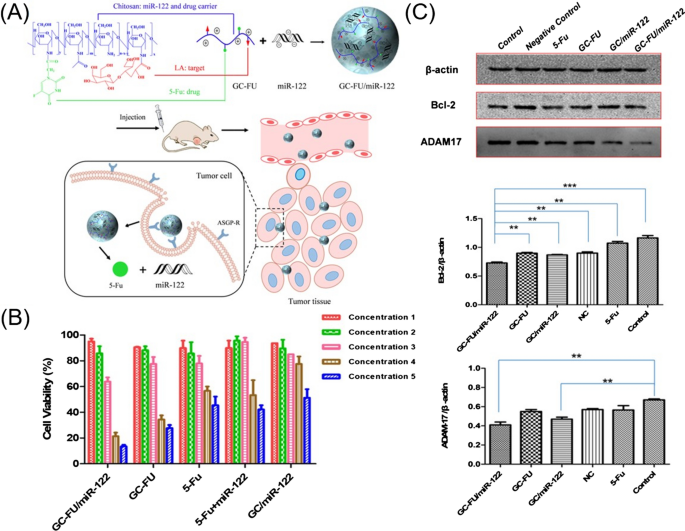
(Reprinted with permission from Ref. [184], Copyright 2019, American Chemical Society)
A Preparation of GC-FU/miR-122 and hepatoma-targeted codelivery of miR-122 and 5-Fu. B Viability of HepG2 cells after incubation with various treatment constructs. C The expression of Bcl-2 and ADAM17 in the HepG2 cells and quantitative analysis.
Host-versus-graft disease
LT is the only effective treatment method for end-stage hepatic diseases, such as acute hepatic failure, a life-threatening systemic complication of hepatic disease, a hepatic-based metabolic disorder, or cirrhosis with complications including HCC, variceal haemorrhage induced by portal hypertension, ascites, hepatorenal syndrome and encephalopathy [186, 187]. However, HVGD, which is the most common type of immune rejection after LT, can lead to abnormal liver function and even graft dysfunction [188, 189]. Therefore, the lifelong use of immunosuppressants is necessary for patients to inhibit transplant rejection. The commonly used immunosuppressants include calcineurin inhibitors (cyclosporine (CsA) and tacrolimus (Tac)), glucocorticoids, cytostatics (azathioprine (AZA) and mycophenolate mofetil (MMF)), and mTOR inhibitors (sirolimus (SRL) and everolimus (EVR)) [187]. Nevertheless, immunosuppressants exhibit certain problems, such as a narrow therapeutic window, poor aqueous solubility, low bioavailability, and significant individual differences. In addition, immunosuppressants can cause severe side effects, such as infection, nephrotoxicity, hepatotoxicity, de novo tumours, pneumonitis, and bone marrow suppression [187, 190]. Nanocarriers can effectively improve the physicochemical properties of immunosuppressants, mediate the targeted delivery of immunosuppressive agents to liver tissues, and reduce the side effects of the agents. Recent development of polymer carriers for the delivery of immunosuppressants is discussed in this section.
-
(i)
Immunosuppressant delivery: Tac blocks the production of interleukin-2 (IL-2), inhibiting T-cell activation and proliferation at an early stage. However, the poor solubility and instability of Tac hinders its clinical use. Therefore, Tac-loaded micelles were developed with various biodegradable polymers, such as poly(ɛ-caprolactone)-PEG-poly(ɛ-caprolactone), PEG-poly(epsilon-caprolactone), PEG-poly(d,l-lactide), poly(methyl vinyl ether-co-maleic anhydride)-graft-hydroxypropyl-β-cyclodextrin, and an ethyl cellulose (EC) polymer, to improve the solubility, bioavailability, and blood circulation of Tac [191,192,193,194,195,196]. MMF is an antiproliferative immunosuppressant drug that can be converted to the active ingredient mycophenolic acid (MPA) in the liver and intestinal wall. A high MPA dose can lead to adverse effects such as diarrhoea, nausea, and vomiting. Mohammed et al. developed an oral formulation of MMF for once-daily dosing using CS-coated PLA or PLGA NPs, and they showed higher envelopment and drug release rates [197]. HVGD after LT is an immune disease mediated by T cells. Therefore, targeted delivery of Tac to T cells leads to precise intervention. A targeted delivery platform based on PLGA NPs decorated with CS and the CD8AP17s aptamer (Apt) was designed to efficiently transport Tac into MOLT-4 cells and reduce off-target cell toxicity [130].
-
(ii)
Nucleic acid delivery: Toll-like receptors (TLRs) play crucial roles in the induction of allograft rejection. Myeloid differentiation factor 88 (MyD88) is a fundamental adaptor in TLR signalling, and inhibiting the expression of MyD88 can prolong the survival of allografts. Therefore, Hu et al. developed a histidine-grafted poly(β-amino ester) (HGPAE) nanocarrier to deliver a plasmid containing MyD88-targeting short hairpin RNA (shRNA). The pMyD88/HGPAE complexes significantly inhibited the expression of MyD88 in rat hepatic tissue, prolonged allograft survival and significantly reduced the serum levels of IL-2 and IFN-γ in the recipients [127].
The fate of NPs in the liver
As NP-based technologies continue to be developed for the diagnosis and therapy of liver diseases [8, 11, 12], the need to understand the intrahepatic distribution and potential clearance mechanisms of NPs is ongoing. The liver, as the largest organ RES in the body, can sequester 30–99% of NPs delivered through the blood circulation system [38]. The fate of these NPs, as reported, includes (i) cellular uptake by KCs. KCs form the first line of liver defence and contribute to substance clearance through the action of scavenger receptors [198]. Therefore, KCs are more likely than others to phagocytose NPs [199, 200]. Once being internalized, NPs may be processed by autophagy and endolysosomal pathways. Autophagy and endolysosomal pathways effectively isolate particles from the surrounding environment, degrade the nanomaterials, reduce the associated toxicity and aid in decreasing cellular stress [201]; (ii) Hepatobiliary elimination. Circulating NPs that are not internalized by KCs or that escape from KCs but are smaller than the diameter of liver sinusoidal fenestrations (up to 100–200 nm), can enter into the space of Disse through the fenestrae or LSECs. Subsequently, they are phagocytosed by hepatocytes, degraded by various enzyme systems, and excreted into the biliary tract [38, 202, 203] (Fig. 9).
However, the distribution and metabolism of NPs change due to the changes of liver anatomy and function in some liver diseases. For example, owing to the capillarization of sinusoids and deposition of ECM in liver fibrosis and cirrhosis, NPs cannot readily enter the space of Disse and thus cannot be taken up by hepatocytes or HSCs to exert therapeutic functions [7, 153]. In contrast, NPs can accumulate in liver tumour by EPR effect as mentioned above and then can be internalized by tumour cells. Therefore, to develop effective nanomedicines to treat certain liver diseases, the relationships between NPs and the liver from an organ-to-cell perspective need a deeper investigation.

(Reprinted with permission from Ref. [200], Copyright 2019, American Chemical Society)
Proposed elimination mechanism of biodegradable nanoparticle (NP) in the liver. Intravenously injected NPs enter the liver and into hepatic sinusoids. A Kupfer cells (KCs) take up the majority of circulating NPs on the basis of NP size. B NPs can escape from KCs. C, D NPs that are smaller than the diameter of liver sinusoidal fenestrations (up to 100–200 nm) can enter the space of Disse through LSECs or fenestrations. E NPs then collect in the space of Disse, where hepatocytes slowly internalize and process them for transport into the bile canaliculus. F Larger NPs may not be able to enter fenestrations or access by LSECs and thus continue to circulate throughout the body.
Conclusions and prospects
Liver diseases pose a serious threat to human health. Nevertheless, conventional medications cannot be delivered to the location of diseases at a sufficient concentration and may cause significant adverse effects. In recent years, various polymer-based nanomedicines have been designed and developed to carry small molecules, peptides and nucleic acids for the diagnosis and treatment of hepatic diseases, resulting in improved therapeutic outcomes and alleviated systemic toxicity. However, the clinical application of polymer nanomedicines is still limited. Several challenges hinder the clinical translation of polymeric nanomedicines and need to be addressed.
First, many potential therapies have been effective in animal models, but their therapeutic efficacy in humans has been less than satisfactory. Several issues may contribute to these outcomes: (i) The types and number of receptors on liver cells are not entirely known, and ligands applied in animal experiments may not specifically recognize human receptors; (ii) NPs, as exogenous substances, exert immunogenetic effects after entering the systemic circulation and are readily internalized by immune cells; hence, it is difficult to deliver drugs to target cells where they can exert their pharmacological effects; and (iii) the animal models cannot mimic the clinical situation. In humans, fibrosis, cirrhosis, and even HCC develop over decades, and symptoms primarily develop in the mid and late stages of the disease. However, these processes develop in weeks or months in rodents, and therefore, the pathology has less time to ‘mature’. More importantly, many treatment experiments have been conducted at earlier stages of the disease, and it seems that diseases in rodents are cured more easily than human liver diseases. Therefore, improved animal models need to be established to better investigate the therapeutic effects of nanomedicines.
Second, polymeric micelles are promising platforms to improve the bioavailability of free drugs. However, after being administered into the bloodstream, micelles are affected by a variety of factors, including temperature, pH, ionic strength, and biological molecules, leading to the instability of the micelle structure [204]. Therefore, it is necessary to improve the stability of micelles to reduce premature agent release in the bloodstream. Studies have shown that dynamic covalent or noncovalent crosslinking can effectively reinforce the structure and improve the stability of micelles [205, 206]; therefore, crosslinked polymer micelles are expected to be promising delivery systems. Improving the controllability of the molecular structure and optimizing the preparation methods of polymer micelles are hotspots for future research. In summary, the development of polymeric carriers with novel materials and technologies is critical, and investigations into the best mechanism for fabrication of micelles as efficient drug delivery systems are important.
Third, a major challenge involves the failure of many NPs to reach targeted liver cells. The liver is the largest organ RES and can sequester 30–99% of administered NPs in the blood circulatory system. However, most NPs, as xenobiotics, are located in KCs, even when targeted ligands are added to the NPs. In addition, some NPs, which escape from KCs, enter the space of Disse and are phagocytized by hepatocytes or HSCs. However, we do not know whether these NPs are deformed or fragmented as they pass through the fenestrations. Therefore, the mechanism by which NPs extravasate into the space of Disse needs further investigation. To reduce the uptake of NPs by the RES and prolong the circulation of the NPs in the bloodstream, PEG chains have been widely used to modify the nanomedicine surface [207, 208]. In addition, although most ligand-modified NPs can be internalized by major target cells in vitro and can accumulate in the liver in vivo, no direct evidence has shown how these NPs can be phagocytized by target cells in vivo and perform effectively. Therefore, interest in using polymers to transport contrast agents for MRI and computed tomography (CT) is increasing, and nanocarriers can co-deliver SPIO and therapeutic agents, enabling dynamic monitoring of the location and effects of nanodrugs [166, 209]. This direction aligns with the current trend towards personalized medicine, where the best treatment schedule and dose vary by individual patient. In addition, the application of radiolabelled constructs and immunohistochemical analyses of tissues from multiple organs can be used to track the trajectory of NPs. Therefore, the application of biodegradable multifunctional composite nanocarriers, with the addition of rigid security and efficacy testing, may provide accurate and effective treatment for hepatic diseases and will be a significant direction for future nanomedicine studies.
Fourth, liver cell-targeted delivery and stimulus-responsive release have become important research aspects in the field of drug delivery. As mentioned above, each disease is characterized by target cells, and nearly all resident liver cells can be reached by applying different carriers. However, the liver, as the largest organ RES, can take up most NPs, and therefore, it is essential to verify that targeted NPs confer better healing than untargeted NPs in vivo. In addition, developing environment-responsive nanomedicines, which can intelligently respond to endogenous (redox, pH, or enzyme) or exogenous stimuli (temperature, ultraviolet light, near infrared light, ultrasound, or magnetic fields) and release payload at targeted sites, will increase therapeutic efficacy for diseased/damaged cells and reduce toxicity to normal cells [210]. Active targeting and environment responsiveness can realize the spatio-temporally controlled drug release, which will be the hotspots in future nanomedicine research. To advance the development of nanomedicine, we need to pay greater attention to the pathophysiology of liver disease and develop reasonable drug carriers capable of differentiating between normal cells and diseased/damaged cells at both the cellular and gene levels. To gain the needed understanding, the joint efforts of chemists, pathologists, physiologists, and other professionals are required.
Finally, rigorous biosafety assessments need to be established to evaluate the immunogenicity of polymer NPs, the safety of NP degradation products, NP effects on hepatocyte functions, and the pharmacokinetics of the medicinal systems. The long-term effects of NPs need to be carefully and systemically evaluated because patients with liver diseases present with low immunity and lack of self-repair ability. Therefore, the safety risks involved in the application of NPs for the treatment of hepatic disease need to be given great attention. Furthermore, liver diseases have a long course, and sensitive methods to continuously monitor changes in liver function during disease treatment are lacking; therefore, sensitive serum markers that can be used to assess the progress of disease and measure the effectiveness of drugs need to be extensively explored.
With the advances in chemistry, biology, nanotechnology and medicine, deeper insights into the pathophysiology of liver diseases, and the emergence of new analysis methods and design concepts, we believe that novel polymeric nanomedicines will be developed to address the aforementioned challenges. As a result, the therapeutic outcomes of polymeric nanomedicines are expected to be improved, thus promoting their clinical translation in the treatment of hepatic diseases.
Availability of data and materials
Not applicable, please refer to the original references.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- HBV:
-
Hepatitis B virus
- LT:
-
Liver transplantation
- NPs:
-
Nanoparticles
- NASH:
-
Nonalcoholic steatohepatitis
- HVGD:
-
Host-versus-graft disease
- PLGA:
-
Poly(lactic-co-glycolic acid)
- NPCs:
-
Nonparenchymal cell
- LSECs:
-
Liver sinusoidal endothelial cells
- HSCs:
-
Hepatic stellate cells
- KCs:
-
Kupffer cells
- NAFLD:
-
Nonalcoholic fatty liver disease
- RES:
-
Reticuloendothelial system
- ECM:
-
Extracellular matrix
- aHSCs:
-
Activated HSCs
- PEG:
-
Polyethylene glycol
- EPR:
-
Enhanced permeability and retention
- HBsAg:
-
Hepatitis B surface antigen
- CS:
-
Chitosan
- PCL:
-
Poly-ε-caprolactone
- BMDCs:
-
Bone marrow-derived dendritic cells
- Ngs:
-
Nanogels
- γ-PGA:
-
Poly-γ-glutamic acid
- pDNA:
-
Plasmid DNA
- CPPs:
-
Cell-penetrating peptides
- PNAs:
-
Peptide nucleic acids
- CSSO:
-
CS oligosaccharide-SS-octadecylamine
- LPH:
-
Lipid polymer hybrid
- NAFL:
-
Nonalcoholic fatty liver
- Res:
-
Resveratrol
- Gal:
-
Galactose
- DNL:
-
De novo lipogenesis
- SREBP:
-
Sterol regulatory element-binding protein
- RAPA:
-
Rapamycin
- FNB:
-
Fenofibrate
- NFD:
-
Nifedipine
- HFD:
-
High-fat diet
- Lac-PDMAEMA:
-
Lactosylated poly(N,N-dimethylaminoethyl methacrylate)
- PPARγ:
-
Peroxisome proliferator-activated receptorγ
- ROS:
-
Reactive oxygen species
- TIMPs:
-
Tissue inhibitors of metalloproteinases
- HMFs:
-
Hepatic myofibroblasts
- mAbs:
-
Monoclonal antibodies
- HA:
-
Hyaluronic acid
- SLB:
-
Silibinin
- P(PBEM-co-DPA):
-
Poly(2-((((4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzyl)oxy) carbonyl) oxy)ethyl methacrylate co 2-(diisopropyl amino)ethyl methacrylate)
- SA:
-
Stearic acid
- TKIs:
-
Tyrosine kinase inhibitors
- VA:
-
Vitamin A
- miRNAs:
-
MicroRNAs
- MRI:
-
Magnetic resonance imaging
- RcP:
-
Retinol-conjugated polyetherimine
- RBP:
-
Retinol binding protein 4
- PSPE:
-
Polyspermine
- cRGD:
-
Cyclic RGD
- TACE:
-
Transarterial chemoembolization
- ICIs:
-
Immune checkpoint inhibitors
- CTLA4:
-
Cytotoxic T-lymphocyte antigen 4
- CPT:
-
Camptothecin
- MMP-9:
-
Matrix metalloproteinase-9
- PLL:
-
Poly-l-lysine
- DOX:
-
Doxorubicin
- ATRA:
-
All-trans retinoic acid
- PCL:
-
Poly(caprolactone)
- PZLL:
-
Poly-(N-ε-carbobenzyloxy-l-lysine)
- SPIO:
-
Superparamagnetic iron oxide
- PEI:
-
Polyethylenimine
- SFB:
-
Sorafenib
- 5-Fu:
-
5-fluorouracil
- P-gp:
-
P-glycoprotein
- PTX:
-
Paclitaxel
- PDMAEMA:
-
Poly(2-(dimethylamino)ethyl methacrylate)
- CsA:
-
Cyclosporine
- Tac:
-
Tacrolimus
- AZA:
-
Azathioprine
- MMF:
-
Mycophenolate mofetil
- SRL:
-
Sirolimus
- EVR:
-
Everolimus
- MPA:
-
Mycophenolic acid
- Apt:
-
Aptamer
- TLRs:
-
Toll-like receptors
- MyD88:
-
Myeloid differentiation factor 88
- HGPAE:
-
Histidine-grafted poly(β-amino ester)
- shRNA:
-
Short hairpin RNA
- CT:
-
Computed tomography
References
Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151–71.
Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6.
Singh L, Indermun S, Govender M, Kumar P, du Toit LC, Choonara YE, et al. Drug delivery strategies for antivirals against hepatitis B virus. Viruses. 2018;10(5):267.
Geissler EK, Schlitt HJ. Immunosuppression for liver transplantation. Gut. 2009;58(3):452–63.
Lohitesh K, Chowdhury R, Mukherjee S. Resistance a major hindrance to chemotherapy in hepatocellular carcinoma: an insight. Cancer Cell Int. 2018;18:44.
Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic steatohepatitis: a review. JAMA. 2020;323(12):1175–83.
Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115(2):209–18.
Reddy LH, Couvreur P. Nanotechnology for therapy and imaging of liver diseases. J Hepatol. 2011;55(6):1461–6.
Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–24.
Gregoriadis G, Wills EJ, Swain CP, Tavill AS. Drug-carrier potential of liposomes in cancer chemotherapy. Lancet. 1974;1(7870):1313–6.
Li L, Wang H, Ong ZY, Xu K, Ee PLR, Zheng S, et al. Polymer- and lipid-based nanoparticle therapeutics for the treatment of liver diseases. Nano Today. 2010;5(4):296–312.
Bottger R, Pauli G, Chao PH, Al Fayez N, Hohenwarter L, Li SD. Lipid-based nanoparticle technologies for liver targeting. Adv Drug Deliv Rev. 2020;154–155:79–101.
Couvreur P, Vauthier C. Nanotechnology: intelligent design to treat complex disease. Pharm Res. 2006;23(7):1417–50.
Ma Z, Zhang B, Fan Y, Wang M, Kebebe D, Li J, et al. Traditional chinese medicine combined with hepatic targeted drug delivery systems: a new strategy for the treatment of liver diseases. Biomed Pharmacother. 2019;117:109128.
Siepmann J, Faham A, Clas SD, Boyd BJ, Jannin V, Bernkop-Schnürch A, et al. Lipids and polymers in pharmaceutical technology: lifelong companions. Int J Pharm. 2019;558:128–42.
Zhu X, Anquillare ELB, Farokhzad OC, Shi J. Chapter 22-polymer- and protein-based nanotechnologies for cancer theranostics. In: Chen X, Wong S, editors. Cancer theranostics. Oxford: Academic Press; 2014. p. 419–36.
Kamaly N, Xiao ZY, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012;41(7):2971–3010.
Farokhzad OC, Langer R. Nanomedicine: developing smarter therapeutic and diagnostic modalities. Adv Drug Deliv Rev. 2006;58(14):1456–9.
Seymour LW, Ferry DR, Anderson D, Hesslewood S, Julyan PJ, Poyner R, et al. Hepatic drug targeting: phase I evaluation of polymer-bound doxorubicin. J Clin Oncol. 2002;20(6):1668–76.
Zhou Q, Sun X, Zeng L, Liu J, Zhang Z. A randomized multicenter phase II clinical trial of mitoxantrone-loaded nanoparticles in the treatment of 108 patients with unresected hepatocellular carcinoma. Nanomedicine. 2009;5(4):419–23.
Jazayeri-Tehrani SA, Rezayat SM, Mansouri S, Qorbani M, Alavian SM, Daneshi-Maskooni M, et al. Nano-curcumin improves glucose indices, lipids, inflammation, and Nesfatin in overweight and obese patients with non-alcoholic fatty liver disease (NAFLD): a double-blind randomized placebo-controlled clinical trial. Nutr Metab. 2019;16:8.
Abdel-Misih SRZ, Bloomston M. Liver anatomy. Surg Clin N Am. 2010;90(4):643–53.
Zhou Z, Xu MJ, Gao B. Hepatocytes: a key cell type for innate immunity. Cell Mol Immunol. 2016;13(3):301–15.
Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43(2 Suppl 1):54–62.
Sørensen KK, Simon-Santamaria J, McCuskey RS, Smedsrød B. Liver sinusoidal endothelial cells. Compr Physiol. 2015;5(4):1751–74.
Braet F, Wisse E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp Hepatol. 2002;1(1):1.
Tamura R, Uemoto S, Tabata Y. Augmented liver targeting of exosomes by surface modification with cationized pullulan. Acta Biomater. 2017;57:274–84.
Kim SI, Shin D, Choi TH, Lee JC, Cheon G-J, Kim K-Y, et al. Systemic and specific delivery of small interfering RNAs to the liver mediated by apolipoprotein A-I. Mol Ther. 2007;15(6):1145–52.
Yang T, Lan Y, Cao M, Ma X, Cao A, Sun Y, et al. Glycyrrhetinic acid-conjugated polymeric prodrug micelles co-delivered with doxorubicin as combination therapy treatment for liver cancer. Colloids Surf B Biointerfaces. 2019;175:106–15.
McCuskey RS. The hepatic microvascular system in health and its response to toxicants. Anat Rec (Hoboken). 2008;291(6):661–71.
Trefts E, Gannon M, Wasserman DH. The liver. Curr Biol. 2017;27(21):R1147-51.
Lachman N, Pawlina W. The liver and biliary apparatus: basic structural anatomy and variations. In: Nicholas J, Talley MDP, Keith D. Lindor MD, Hugo E. Vargas MD, editors. Practical gastroenterology and hepatology: liver and biliary disease. Oxford: Blackwell Publishing Ltd; 2010. pp. 1–16.
Walkey CD, Chan WC. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem Soc Rev. 2012;41(7):2780–99.
Cheng SH, Li FC, Souris JS, Yang CS, Tseng FG, Lee HS, et al. Visualizing dynamics of sub-hepatic distribution of nanoparticles using intravital multiphoton fluorescence microscopy. ACS Nano. 2012;6(5):4122–31.
Ogawara K, Yoshida M, Higaki K, Kimura T, Shiraishi K, Nishikawa M, et al. Hepatic uptake of polystyrene microspheres in rats: effect of particle size on intrahepatic distribution. J Control Release. 1999;59(1):15–22.
Romero EL, Morilla MJ, Regts J, Koning GA, Scherphof GL. On the mechanism of hepatic transendothelial passage of large liposomes. FEBS Lett. 1999;448(1):193–6.
Champion JA, Mitragotri S. Shape induced inhibition of phagocytosis of polymer particles. Pharm Res. 2009;26(1):244–9.
Zhang YN, Poon W, Tavares AJ, McGilvray ID, Chan WCW. Nanoparticle-liver interactions: cellular uptake and hepatobiliary elimination. J Control Release. 2016;240:332–48.
Gu FX, Karnik R, Wang AZ, Alexis F, Levy-Nissenbaum E, Hong S, et al. Targeted nanoparticles for cancer therapy. Nano Today. 2007;2(3):14–21.
Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–92.
Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65(1–2):271–84.
Bertrand N, Wu J, Xu XY, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25.
Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, et al. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55(17):3752–6.
Farra R, Musiani F, Perrone F, Čemažar M, Kamenšek U, Tonon F, et al. Polymer-mediated delivery of siRNAs to Hepatocellular Carcinoma: variables affecting specificity and effectiveness. Molecules. 2018;23(4):777.
Wu H, Wang MD, Liang L, Xing H, Zhang CW, Shen F, et al. Nanotechnology for hepatocellular carcinoma: from surveillance, diagnosis to management. Small. 2021;17(6):e2005236.
Malla RR, Kumari S, Kgk D, Momin S, Nagaraju GP. Nanotheranostics: their role in hepatocellular carcinoma. Crit Rev Oncol Hematol. 2020;151:102968.
Gaumet M, Vargas A, Gurny R, Delie F. Nanoparticles for drug delivery: the need for precision in reporting particle size parameters. Eur J Pharm Biopharm. 2008;69(1):1–9.
Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev. 2008;60(15):1615–26.
Danhier F, Feron O, Preat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148(2):135–46.
Li J, Zhang Y, Cai C, Rong X, Shao M, Li J, et al. Collaborative assembly of doxorubicin and galactosyl diblock glycopolymers for targeted drug delivery of hepatocellular carcinoma. Biomater Sci. 2020;8(1):189–200.
Zhao J, Yan C, Chen Z, Liu J, Song H, Wang W, et al. Dual-targeting nanoparticles with core-crosslinked and pH/redox-bioresponsive properties for enhanced intracellular drug delivery. J Colloid Interface Sci. 2019;540:66–77.
Kuruvilla SP, Tiruchinapally G, Kaushal N, ElSayed MEH. Effect of N-acetylgalactosamine ligand valency on targeting dendrimers to hepatic cancer cells. Int J Pharm. 2018;545(1–2):27–36.
Detampel P, Witzigmann D, Krähenbühl S, Huwyler J. Hepatocyte targeting using pegylated asialofetuin-conjugated liposomes. J Drug Target. 2014;22(3):232–41.
Zhang Q, Zhang X, Chen T, Wang X, Fu Y, Jin Y, et al. A safe and efficient hepatocyte-selective carrier system based on myristoylated preS1/21–47 domain of hepatitis B virus. Nanoscale. 2015;7(20):9298–310.
Hefnawy A, Khalil IH, Arafa K, Emara M, El-Sherbiny IM. Dual-ligand functionalized core-shell chitosan-based nanocarrier for hepatocellular carcinoma-targeted drug delivery. Int J Nanomed. 2020;15:821–37.
Shen Z, Li B, Liu Y, Zheng G, Guo Y, Zhao R, et al. A self-assembly nanodrug delivery system based on amphiphilic low generations of PAMAM dendrimers-ursolic acid conjugate modified by lactobionic acid for HCC targeting therapy. Nanomedicine. 2018;14(2):227–36.
Wang X, Qi Y, Liu L, Ganbold T, Baigude H, Han J. Preparation and cell activities of lactosylated curdlan-triornithine nanoparticles for enhanced DNA/siRNA delivery in hepatoma cells. Carbohydr Polym. 2019;225:115252.
Qi XR, Yan WW, Shi J. Hepatocytes targeting of cationic liposomes modified with soybean sterylglucoside and polyethylene glycol. World J Gastroenterol. 2005;11(32):4947–52.
Zheng Y, Shi S, Liu Y, Zhao Y, Sun Y. Targeted pharmacokinetics of polymeric micelles modified with glycyrrhetinic acid and hydrazone bond in H22 tumor-bearing mice. J Biomater Appl. 2019;34(1):141–51.
Li ZP, Tian GX, Jiang H, Pan RY, Lian B, Wang M, et al. Liver-targeting and pH-sensitive sulfated hyaluronic acid mixed micelles for hepatoma therapy. Int J Nanomed. 2019;14:9437–52.
Longmuir KJ, Haynes SM, Baratta JL, Kasabwalla N, Robertson RT. Liposomal delivery of doxorubicin to hepatocytes in vivo by targeting heparan sulfate. Int J Pharm. 2009;382(1):222–33.
Akinc A, Querbes W, De S, Qin J, Frank-Kamenetsky M, Jayaprakash KN, et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol Ther. 2010;18(7):1357–64.
Fielding CJ. Lipoprotein receptors, plasma cholesterol metabolism, and the regulation of cellular free cholesterol concentration. FASEB J. 1992;6(13):3162–8.
Jędrzak A, Grześkowiak BF, Golba K, Coy E, Synoradzki K, Jurga S, et al. Magnetite nanoparticles and spheres for chemo- and photothermal therapy of hepatocellular carcinoma in vitro. Int J Nanomed. 2020;15:7923–36.
Koirala N, Das D, Fayazzadeh E, Sen S, McClain A, Puskas JE, et al. Folic acid conjugated polymeric drug delivery vehicle for targeted cancer detection in hepatocellular carcinoma. J Biomed Mater Res A. 2019;107(11):2522–35.
Gao D-Y, Lin T-T, Sung Y-C, Liu YC, Chiang W-H, Chang C-C, et al. CXCR4-targeted lipid-coated PLGA nanoparticles deliver sorafenib and overcome acquired drug resistance in liver cancer. Biomaterials. 2015;67:194–203.
Kumari P, Rompicharla SVK, Muddineti OS, Ghosh B, Biswas S. Transferrin-anchored poly(lactide) based micelles to improve anticancer activity of curcumin in hepatic and cervical cancer cell monolayers and 3D spheroids. Int J Biol Macromol. 2018;116:1196–213.
Yang H, Miao Y, Chen L, Li Z, Yang R, Xu X, et al. Redox-responsive nanoparticles from disulfide bond-linked poly-(N-ε-carbobenzyloxy-l-lysine)-grafted hyaluronan copolymers as theranostic nanoparticles for tumor-targeted MRI and chemotherapy. Int J Biol Macromol. 2020;148:483–92.
Akhter A, Hayashi Y, Sakurai Y, Ohga N, Hida K, Harashima H. Ligand density at the surface of a nanoparticle and different uptake mechanism: two important factors for successful siRNA delivery to liver endothelial cells. Int J Pharm. 2014;475(1):227–37.
Kamps JA, Morselt HW, Swart PJ, Meijer DK, Scherphof GL. Massive targeting of liposomes, surface-modified with anionized albumins, to hepatic endothelial cells. Proc Natl Acad Sci USA. 1997;94(21):11681–5.
Van Berkel TJ, De Rijke YB, Kruijt JK. Different fate in vivo of oxidatively modified low density lipoprotein and acetylated low density lipoprotein in rats. Recognition by various scavenger receptors on Kupffer and endothelial liver cells. J Biol Chem. 1991;266(4):2282–9.
Toriyabe N, Hayashi Y, Hyodo M, Harashima H. Synthesis and evaluation of stearylated hyaluronic acid for the active delivery of liposomes to liver endothelial cells. Biol Pharm Bull. 2011;34(7):1084–9.
Praaning-van Dalen DP, de Leeuw AM, Brouwer A, Knook DL. Rat liver endothelial cells have a greater capacity than Kupffer cells to endocytose N-acetylglucosamine- and mannose-terminated glycoproteins. Hepatology. 1987;7(4):672–9.
Sano A, Taylor ME, Leaning MS, Summerfield JA. Uptake and processing of glycoproteins by isolated rat hepatic endothelial and kupffer cells. J Hepatol. 1990;10(2):211–6.
Malovic I, Sørensen KK, Elvevold KH, Nedredal GI, Paulsen S, Erofeev AV, et al. The mannose receptor on murine liver sinusoidal endothelial cells is the main denatured collagen clearance receptor. Hepatology. 2007;45(6):1454–61.
Fridman WH. Fc receptors and immunoglobulin binding factors. FASEB J. 1991;5(12):2684–90.
Zhu J, Qin F, Ji Z, Fei W, Tan Z, Hu Y, et al. Mannose-modified PLGA nanoparticles for sustained and targeted delivery in hepatitis B virus immunoprophylaxis. AAPS PharmSciTech. 2019;21(1):13.
Lai C, Li C, Luo X, Liu M, Liu X, Hu L, et al. Use of dual-ligand modification in Kupffer cell-targeted liposomes to examine the contribution of Kupffer cells to accelerated blood clearance phenomenon. Mol Pharm. 2018;15(7):2548–58.
Higuchi Y, Kawakami S, Yamashita F, Hashida M. The potential role of fucosylated cationic liposome/NFκB decoy complexes in the treatment of cytokine-related liver disease. Biomaterials. 2007;28(3):532–9.
Shimada K, Kamps JAAM, Regts J, Ikeda K, Shiozawa T, Hirota S, et al. Biodistribution of liposomes containing synthetic galactose-terminated diacylglyceryl-poly(ethyleneglycol)s. Biochim Biophys Acta (BBA) Biomembr. 1997;1326(2):329–41.
Rafique A, Etzerodt A, Graversen JH, Moestrup SK, Dagnæs-Hansen F, Møller HJ. Targeted lipid nanoparticle delivery of calcitriol to human monocyte-derived macrophages in vitro and in vivo: investigation of the anti-inflammatory effects of calcitriol. Int J Nanomed. 2019;14:2829–46.
Koning GA, Morselt HW, Gorter A, Allen TM, Zalipsky S, Scherphof GL, et al. Interaction of differently designed immunoliposomes with colon cancer cells and kupffer cells. An in vitro comparison. Pharm Res. 2003;20(8):1249–57.
Helmy KY, Katschke KJ Jr, Gorgani NN, Kljavin NM, Elliott JM, Diehl L, et al. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell. 2006;124(5):915–27.
Dutta R, Kumar V, Peng Y, Evande RE, Grem JL, Mahato RI. Pharmacokinetics and biodistribution of GDC-0449 loaded micelles in normal and liver fibrotic mice. Pharm Res. 2017;34(3):564–78.
Yildirim T, Matthäus C, Press AT, Schubert S, Bauer M, Popp J, et al. Uptake of retinoic acid-modified PMMA nanoparticles in LX-2 and liver tissue by Raman Imaging and Intravital Microscopy. Macromol Biosci. 2017;17(10):1700064.
Qiao JB, Fan QQ, Xing L, Cui PF, He YJ, Zhu JC, et al. Vitamin A-decorated biocompatible micelles for chemogene therapy of liver fibrosis. J Control Release. 2018;283:113–25.
El-Mezayen NS, El-Hadidy WF, El-Refaie WM, Shalaby TI, Khattab MM, El-Khatib AS. Hepatic stellate cell-targeted imatinib nanomedicine versus conventional imatinib: a novel strategy with potent efficacy in experimental liver fibrosis. J Control Release. 2017;266:226–37.
Ji D, Wang Q, Zhao Q, Tong H, Yu M, Wang M, et al. Co-delivery of miR-29b and germacrone based on cyclic RGD-modified nanoparticles for liver fibrosis therapy. J Nanobiotechnol. 2020;18(1):86.
Yang J, Hou Y, Ji G, Song Z, Liu Y, Dai G, et al. Targeted delivery of the RGD-labeled biodegradable polymersomes loaded with the hydrophilic drug oxymatrine on cultured hepatic stellate cells and liver fibrosis in rats. Eur J Pharm Sci. 2014;52:180–90.
van Dijk F, Teekamp N, Beljaars L, Post E, Zuidema J, Steendam R, et al. Pharmacokinetics of a sustained release formulation of PDGFβ-receptor directed carrier proteins to target the fibrotic liver. J Control Release. 2018;269:258–65.
van Dijk F, Teekamp N, Post E, Schuppan D, Kim YO, Zuidema J, et al. The antifibrotic potential of a sustained release formulation of a PDGFβ-receptor targeted rho kinase inhibitor. J Control Release. 2019;296:250–7.
Adrian JE, Poelstra K, Scherphof GL, Molema G, Meijer DK, Reker-Smit C, et al. Interaction of targeted liposomes with primary cultured hepatic stellate cells: involvement of multiple receptor systems. J Hepatol. 2006;44(3):560–7.
Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol. 2017;17(5):306–21.
Hammoutene A, Rautou PE. Role of liver sinusoidal endothelial cells in non-alcoholic fatty liver disease. J Hepatol. 2019;70(6):1278–91.
Yin C, Evason KJ, Asahina K, Stainier DY. Hepatic stellate cells in liver development, regeneration, and cancer. J Clin Invest. 2013;123(5):1902–10.
Gissen P, Arias IM. Structural and functional hepatocyte polarity and liver disease. J Hepatol. 2015;63(4):1023–37.
Subbiah R, Ramalingam P, Ramasundaram S, Kim DY, Park K, Ramasamy MK, et al. N,N,N-Trimethyl chitosan nanoparticles for controlled intranasal delivery of HBV surface antigen. Carbohydr Polym. 2012;89(4):1289–97.
Zeng P, Xu Y, Zeng C, Ren H, Peng M. Chitosan-modified poly(D,L-lactide-co-glycolide) nanospheres for plasmid DNA delivery and HBV gene-silencing. Int J Pharm. 2011;415(1–2):259–66.
Xue M, Hu S, Lu Y, Zhang Y, Jiang X, An S, et al. Development of chitosan nanoparticles as drug delivery system for a prototype capsid inhibitor. Int J Pharm. 2015;495(2):771–82.
Miao J, Yang XQ, Gao Z, Li Q, Meng TT, Wu JY, et al. Redox-responsive chitosan oligosaccharide-SS-Octadecylamine polymeric carrier for efficient anti-hepatitis B virus gene therapy. Carbohydr Polym. 2019;212:215–21.
Wang H, Han Q, Zhao H, Xu D, Zhang J. Single dose HBsAg CS-γ-PGA nanogels induce potent protective immune responses against HBV infection. Eur J Pharm Biopharm. 2018;124:82–8.
Zhao R, Zhu M, Zhou S, Feng W, Chen H. Rapamycin-loaded mPEG-PLGA nanoparticles ameliorate hepatic steatosis and liver injury in non-alcoholic fatty liver disease. Front Chem. 2020;8:407.
Zai W, Chen W, Wu Z, Jin X, Fan J, Zhang X, et al. Targeted interleukin-22 gene delivery in the liver by Polymetformin and Penetratin-Based hybrid nanoparticles to treat nonalcoholic fatty liver disease. ACS Appl Mater Interfaces. 2019;11(5):4842–57.
Teng W, Zhao L, Yang S, Zhang C, Liu M, Luo J, et al. The hepatic-targeted, resveratrol loaded nanoparticles for relief of high fat diet-induced nonalcoholic fatty liver disease. J Control Release. 2019;307:139–49.
Lee S, Han D, Kang HG, Jeong SJ, Jo JE, Shin J, et al. Intravenous sustained-release nifedipine ameliorates nonalcoholic fatty liver disease by restoring autophagic clearance. Biomaterials. 2019;197:1–11.
Wan SQ, Zhang L, Quan YY, Wei K. Resveratrol-loaded PLGA nanoparticles: enhanced stability, solubility and bioactivity of resveratrol for non-alcoholic fatty liver disease therapy. R Soc Open Sci. 2018;5(11):181457.
Kurniawan DW, Jajoriya AK, Dhawan G, Mishra D, Argemi J, Bataller R, et al. Therapeutic inhibition of spleen tyrosine kinase in inflammatory macrophages using PLGA nanoparticles for the treatment of non-alcoholic steatohepatitis. J Control Release. 2018;288:227–38.
He S, Guo W, Deng F, Chen K, Jiang Y, Dong M, et al. Targeted delivery of microRNA 146b mimic to hepatocytes by lactosylated PDMAEMA nanoparticles for the treatment of NAFLD. Artif Cells Nanomed Biotechnol. 2018;46(sup2):217–28.
Cao YN, Baiyisaiti A, Wong CW, Hsu SH, Qi R. Polyurethane nanoparticle-loaded fenofibrate exerts inhibitory effects on nonalcoholic fatty liver disease in mice. Mol Pharm. 2018;15(10):4550–7.
Shafie F, Nabavizadeh F, Shafie Ardestani M, Panahi M, Adeli S, Samandari H, et al. Sorafenib-loaded PAMAM dendrimer attenuates liver fibrosis and its complications in bile-duct-ligated rats. Can J Physiol Pharmacol. 2019;97(8):691–8.
Krithika R, Vhora I, Verma RJ. Preparation, toxicity analysis and in vivo protective effect of phyllanthin-loaded PLGA nanoparticles against CCl4-induced hepatic fibrosis. J Drug Deliv Sci Technol. 2019;51:364–71.
Hassan R, Tammam SN, Safy SE, Abdel-Halim M, Asimakopoulou A, Weiskirchen R, et al. Prevention of hepatic stellate cell activation using JQ1- and atorvastatin-loaded chitosan nanoparticles as a promising approach in therapy of liver fibrosis. Eur J Pharm Biopharm. 2019;134:96–106.
El-Safy S, Tammam SN, Abdel-Halim M, Ali ME, Youshia J, Shetab Boushehri MA, et al. Collagenase loaded chitosan nanoparticles for digestion of the collagenous scar in liver fibrosis: the effect of chitosan intrinsic collagen binding on the success of targeting. Eur J Pharm Biopharm. 2020;148:54–66.
Chang CC, Yang Y, Gao DY, Cheng HT, Hoang B, Chao PH, et al. Docetaxel-carboxymethylcellulose nanoparticles ameliorate CCl(4)-induced hepatic fibrosis in mice. J Drug Target. 2018;26(5–6):516–24.
Younis N, Shaheen MA, Abdallah MH. Silymarin-loaded Eudragit(®) RS100 nanoparticles improved the ability of silymarin to resolve hepatic fibrosis in bile duct ligated rats. Biomed Pharmacother. 2016;81:93–103.
Thomas RG, Moon MJ, Kim JH, Lee JH, Jeong YY. Effectiveness of losartan-loaded hyaluronic acid (HA) Micelles for the reduction of advanced hepatic fibrosis in C3H/HeN mice model. PLoS ONE. 2015;10(12):e0145512.
Safer AM, Hanafy NA, Bharali DJ, Cui H, Mousa SA. Effect of green tea extract encapsulated into chitosan nanoparticles on hepatic fibrosis collagen fibers assessed by Atomic Force Microscopy in rat hepatic fibrosis model. J Nanosci Nanotechnol. 2015;15(9):6452–9.
Kaps L, Nuhn L, Aslam M, Brose A, Foerster F, Rosigkeit S, et al. In vivo gene-silencing in fibrotic liver by siRNA-loaded cationic nanohydrogel particles. Adv Healthc Mater. 2015;4(18):2809–15.
Nie X, Liu Y, Li M, Yu X, Yuan W, Huang S, et al. SP94 peptide-functionalized PEG-PLGA nanoparticle loading with cryptotanshinone for targeting therapy of hepatocellular carcinoma. AAPS PharmSciTech. 2020;21(4):124.
Li Z, Ye L, Liu J, Lian D, Li X. Sorafenib-loaded nanoparticles based on biodegradable dendritic polymers for enhanced therapy of hepatocellular carcinoma. Int J Nanomed. 2020;15:1469–80.
Varshosaz J, Raghami F, Rostami M, Jahanian A. PEGylated trimethylchitosan emulsomes conjugated to octreotide for targeted delivery of sorafenib to hepatocellular carcinoma cells of HepG2. J Liposome Res. 2019;29(4):383–98.
Varshosaz J, Sadri F, Rostami M, Mirian M, Taymouri S. Synthesis of pectin-deoxycholic acid conjugate for targeted delivery of anticancer drugs in hepatocellular carcinoma. Int J Biol Macromol. 2019;139:665–77.
Toshiyama R, Konno M, Eguchi H, Takemoto H, Noda T, Asai A, et al. Poly(ethylene glycol)-poly(lysine) block copolymer-ubenimex conjugate targets aminopeptidase N and exerts an antitumor effect in hepatocellular carcinoma stem cells. Oncogene. 2019;38(2):244–60.
Patil S, Ujalambkar V, Rathore A, Rojatkar S, Pokharkar V. Galangin loaded galactosylated pluronic F68 polymeric micelles for liver targeting. Biomed Pharmacother. 2019;112:108691.
Huang Y, Xu Y, Wu Y, Chen T, Lu W, Yu J. Bioinspired nanoplatform for enhanced delivery efficiency of doxorubicin into nucleus with fast endocytosis, lysosomal pH-triggered drug release, and reduced efflux. Colloids Surf B Biointerfaces. 2019;183:110413.
Pandey P, Rahman M, Bhatt PC, Beg S, Paul B, Hafeez A, et al. Implication of nano-antioxidant therapy for treatment of hepatocellular carcinoma using PLGA nanoparticles of rutin. Nanomedicine (Lond). 2018;13(8):849–70.
Hu F, Wang H, Zhang S, Peng Y, Su L, Chang J, et al. Inhibition of myeloid differentiation factor 88 signaling mediated by histidine-grafted poly(β-amino ester) ester nanovector induces donor-specific liver allograft tolerance. Int J Nanomed. 2015;10:4367–82.
Mistry NP, Desai JL, Thakkar HP. Formulation and evaluation of tacrolimus-loaded galactosylated poly(lactic-co-glycolic acid) nanoparticles for liver targeting. J Pharm Pharmacol. 2015;67(10):1337–48.
Azzi J, Yin Q, Uehara M, Ohori S, Tang L, Cai K, et al. Targeted delivery of immunomodulators to lymph nodes. Cell Rep. 2016;15(6):1202–13.
Mansouri A, Abnous K, Alibolandi M, Taghdisi SM, Ramezani M. Targeted delivery of tacrolimus to T cells by pH-responsive aptamer-chitosan- poly(lactic-co-glycolic acid) nanocomplex. J Cell Physiol. 2019;234(10):18262–71.
Asselah T, Loureiro D, Boyer N, Mansouri A. Targets and future direct-acting antiviral approaches to achieve hepatitis B virus cure. Lancet Gastroenterol Hepatol. 2019;4(11):883–92.
Jesus S, Soares E, Costa J, Borchard G, Borges O. Immune response elicited by an intranasally delivered HBsAg low-dose adsorbed to poly-ε-caprolactone based nanoparticles. Int J Pharm. 2016;504(1–2):59–69.
Dewangan HK, Pandey T, Maurya L, Singh S. Rational design and evaluation of HBsAg polymeric nanoparticles as antigen delivery carriers. Int J Biol Macromol. 2018;111:804–12.
Dewangan HK, Pandey T, Singh S. Nanovaccine for immunotherapy and reduced hepatitis-B virus in humanized model. Artif Cells Nanomed Biotechnol. 2018;46(8):2033–42.
Chuan D, Jin T, Fan R, Zhou L, Guo G. Chitosan for gene delivery: methods for improvement and applications. Adv Colloid Interface Sci. 2019;268:25–38.
Ndeboko B, Lemamy GJ, Nielsen PE, Cova L. Therapeutic potential of cell penetrating peptides (CPPs) and cationic polymers for chronic hepatitis B. Int J Mol Sci. 2015;16(12):28230–41.
Ayoub MM, Elantouny NG, El-Nahas HM, Ghazy FES. Injectable PLGA Adefovir microspheres; the way for long term therapy of chronic hepatitis-B. Eur J Pharm Sci. 2018;118:24–31.
Ayoub MM, Jasti B, Elantouny NG, Elnahas H, Ghazy FE. Comparative study of PLGA in-situ implant and nanoparticle formulations of entecavir; in-vitro and in-vivo evaluation. J Drug Deliv Sci Technol. 2020;56:101585.
Zhang C, Wang A, Wang H, Yan M, Liang R, He X, et al. Entecavir-loaded poly (lactic-co-glycolic acid) microspheres for long-term therapy of chronic hepatitis-B: preparation and in vitro and in vivo evaluation. Int J Pharm. 2019;560:27–34.
Hamdi M, Abdel-Bar HM, Elmowafy E, Al-Jamal KT, Awad GAS. An integrated vitamin E-coated polymer hybrid nanoplatform: a lucrative option for an enhanced in vitro macrophage retention for an anti-hepatitis B therapeutic prospect. PLoS ONE. 2020;15(1):e0227231.
Brunt EM, Wong VW, Nobili V, Day CP, Sookoian S, Maher JJ, et al. Nonalcoholic fatty liver disease. Nat Rev Dis Primers. 2015;1:15080.
Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24(7):908–22.
Wang XJ, Malhi H. Nonalcoholic fatty liver disease. Ann Intern Med. 2018;169(9):65–80.
Alonso C, Fernández-Ramos D, Varela-Rey M, Martínez-Arranz I, Navasa N, Van Liempd SM, et al. Metabolomic identification subtypes nonalcoholic steatohepatitis. Gastroenterology. 2017;152(6):1449–61.
Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142(4):711–25.
Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52(2):774–88.
Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the study of Liver Diseases. Hepatology. 2018;67(1):328–57.
Liang J, Liu Y, Liu J, Li Z, Fan Q, Jiang Z, et al. Chitosan-functionalized lipid-polymer hybrid nanoparticles for oral delivery of silymarin and enhanced lipid-lowering effect in NAFLD. J Nanobiotechnol. 2018;16(1):64.
Marcellin P, Kutala BK. Liver diseases: a major, neglected global public health problem requiring urgent actions and large-scale screening. Liver Int. 2018;38(Suppl 1):2–6.
Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut. 2015;64(5):830–41.
Novo E, Parola M. Redox mechanisms in hepatic chronic wound healing and fibrogenesis. Fibrogenesis Tissue Repair. 2008;1(1):5.
Novo E, Cannito S, Paternostro C, Bocca C, Miglietta A, Parola M. Cellular and molecular mechanisms in liver fibrogenesis. Arch Biochem Biophys. 2014;548:20–37.
Parola M, Pinzani M. Liver fibrosis: pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. 2019;65:37–55.
Iredale JP, Thompson A, Henderson NC. Extracellular matrix degradation in liver fibrosis: biochemistry and regulation. Biochim Biophys Acta. 2013;1832(7):876–83.
Kang JH, Toita R, Murata M. Liver cell-targeted delivery of therapeutic molecules. Crit Rev Biotechnol. 2016;36(1):132–43.
Mahdinloo S, Kiaie SH, Amiri A, Hemmati S, Valizadeh H, Zakeri-Milani P. Efficient drug and gene delivery to liver fibrosis: rationale, recent advances, and perspectives. Acta Pharm Sinica B. 2020;10(7):1279–93.
Rohilla R, Garg T, Goyal AK, Rath G. Herbal and polymeric approaches for liver-targeting drug delivery: novel strategies and their significance. Drug Deliv. 2016;23(5):1645–61.
Rockey DC. Current and future anti-fibrotic therapies for chronic liver disease. Clin Liver Dis. 2008;12(4):939–62.
Yoon YJ, Friedman SL, Lee YA. Antifibrotic therapies: where are we now? Semin Liver Dis. 2016;36(1):87–98.
Latief U, Ahmad R. Herbal remedies for liver fibrosis: a review on the mode of action of fifty herbs. J Tradit Complement Med. 2018;8(3):352–60.
Li W, Zhou C, Fu Y, Chen T, Liu X, Zhang Z, et al. Targeted delivery of hyaluronic acid nanomicelles to hepatic stellate cells in hepatic fibrosis rats. Acta Pharm Sin B. 2020;10(4):693–710.
Lin L, Gong H, Li R, Huang J, Cai M, Lan T, et al. Nanodrug with ROS and pH dual-sensitivity ameliorates liver fibrosis via multicellular regulation. Adv Sci (Weinh). 2020;7(7):1903138.
Hu Q, Hu S, Fleming E, Lee JY, Luo Y. Chitosan-caseinate-dextran ternary complex nanoparticles for potential oral delivery of astaxanthin with significantly improved bioactivity. Int J Biol Macromol. 2020;151:747–56.
Lin TT, Gao DY, Liu YC, Sung YC, Wan DH, Liu JY, et al. Development and characterization of sorafenib-loaded PLGA nanoparticles for the systemic treatment of liver fibrosis. J Controlled Release. 2016;221:62–70.
Fan QQ, Zhang CL, Qiao JB, Cui PF, Xing L, Oh YK, et al. Extracellular matrix-penetrating nanodrill micelles for liver fibrosis therapy. Biomaterials. 2020;230:119616.
Wu J, Huang J, Kuang S, Chen J, Li X, Chen B, et al. Synergistic MicroRNA therapy in liver fibrotic rat using MRI-Visible nanocarrier targeting hepatic stellate cells. Adv Sci (Weinh). 2019;6(5):1801809.
Leber N, Kaps L, Aslam M, Schupp J, Brose A, Schäffel D, et al. SiRNA-mediated in vivo gene knockdown by acid-degradable cationic nanohydrogel particles. J Control Release. 2017;248:10–23.
Zhang Z, Wang C, Zha Y, Hu W, Gao Z, Zang Y, et al. Corona-directed nucleic acid delivery into hepatic stellate cells for liver fibrosis therapy. ACS Nano. 2015;9(3):2405–19.
Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–62.
Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao YM, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153(4):996–1005.
Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37(2):429–42.
Elnaggar MH, Abushouk AI, Hassan AHE, Lamloum HM, Benmelouka A, Moatamed SA, et al. Nanomedicine as a putative approach for active targeting of hepatocellular carcinoma. Semin Cancer Biol. 2021;69:91–9.
Huang Y, Zhang W, Xu Y, Zhu S, Wu Y, Chen T, et al. Dynamic core crosslinked camptothecin prodrug micelles with reduction sensitivity and boronic acid-mediated enhanced endocytosis: an intelligent tumor-targeted delivery nanoplatform. Int J Pharm. 2020;580:119250.
Yang DH, Kim HJ, Park K, Kim JK, Chun HJ. Preparation of poly-l-lysine-based nanoparticles with pH-sensitive release of curcumin for targeted imaging and therapy of liver cancer in vitro and in vivo. Drug Deliv. 2018;25(1):950–60.
Yang T, Du G, Cui Y, Yu R, Hua C, Tian W, et al. pH-sensitive doxorubicin-loaded polymeric nanocomplex based on β-cyclodextrin for liver cancer-targeted therapy. Int J Nanomed. 2019;14:1997–2010.
Yang D, Luo W, Wang J, Zheng M, Liao XH, Zhang N, et al. A novel controlled release formulation of the Pin1 inhibitor ATRA to improve liver cancer therapy by simultaneously blocking multiple cancer pathways. J Control Release. 2018;269:405–22.
Gan H, Chen L, Sui X, Wu B, Zou S, Li A, et al. Enhanced delivery of sorafenib with anti-GPC3 antibody-conjugated TPGS-b-PCL/Pluronic P123 polymeric nanoparticles for targeted therapy of hepatocellular carcinoma. Mater Sci Eng C Mater Biol Appl. 2018;91:395–403.
Wang J, Xia Y, Liu H, Xia J, Qian M, Zhang L, et al. Poly(lactobionamidoethyl methacrylate)-based amphiphiles with ultrasound-labile components in manufacture of drug delivery nanoparticulates for augmented cytotoxic efficacy to hepatocellular carcinoma. J Colloid Interface Sci. 2019;551:1–9.
Yan T, Cheng J, Liu Z, Cheng F, Wei X, Huang Y, et al. Acid-sensitive polymeric vector targeting to hepatocarcinoma cells via glycyrrhetinic acid receptor-mediated endocytosis. Mater Sci Eng C Mater Biol Appl. 2018;87:32–40.
Guo H, Xu M, Cao Z, Li W, Chen L, Xie X, et al. Ultrasound-assisted mir-122-loaded polymeric nanodroplets for hepatocellular carcinoma gene therapy. Mol Pharm. 2020;17(2):541–53.
Yang J, Zhang J, Liu Y, Shi Z, Han H, Li Q. Phenylboronic acid-modified polyamidoamine-mediated delivery of short GC rich DNA for hepatocarcinoma gene therapy. Biomater Sci. 2019;7(8):3348–58.
Liu L, Zong ZM, Liu Q, Jiang SS, Zhang Q, Cen LQ, et al. A novel galactose-PEG-conjugated biodegradable copolymer is an efficient gene delivery vector for immunotherapy of hepatocellular carcinoma. Biomaterials. 2018;184:20–30.
Wu B, Li A, Zhang Y, Liu X, Zhou S, Gan H, et al. Resistance of hepatocellular carcinoma to sorafenib can be overcome with co-delivery of PI3K/mTOR inhibitor BEZ235 and sorafenib in nanoparticles. Expert Opin Drug Deliv. 2020;17(4):573–87.
Ning Q, Liu YF, Ye PJ, Gao P, Li ZP, Tang SY, et al. Delivery of liver-specific miRNA-122 using a targeted macromolecular prodrug toward synergistic therapy for hepatocellular carcinoma. ACS Appl Mater Interfaces. 2019;11(11):10578–88.
Cheng H, Wu Z, Wu C, Wang X, Liow SS, Li Z, et al. Overcoming STC2 mediated drug resistance through drug and gene co-delivery by PHB-PDMAEMA cationic polyester in liver cancer cells. Mater Sci Eng C Mater Biol Appl. 2018;83:210–7.
O’Leary JG, Lepe R, Davis GL. Indications for liver transplantation. Gastroenterology. 2008;134(6):1764–76.
European Association for the Study of the Liver. EASL clinical practice guidelines: liver transplantation. J Hepatol. 2016;64(2):433–85.
Afzali B, Lechler RI, Hernandez-Fuentes MP. Allorecognition and the alloresponse: clinical implications. Tissue Antigens. 2007;69(6):545–56.
Banff schema for grading. Liver allograft rejection: an international consensus document. Hepatology. 1997;25(3):658–63.
Halloran PF, Kreepala C, Einecke G, Loupy A, Sellarés J. Therapeutic approaches to organ transplantation. In: Li XC, Jevnikar AM, editors. Transplant immunology. Hoboken: Wiley; 2015.
Wang Y, Wang C, Fu S, Liu Q, Dou D, Lv H, et al. Preparation of Tacrolimus loaded micelles based on poly(ɛ-caprolactone)-poly(ethylene glycol)-poly(ɛ-caprolactone). Int J Pharm. 2011;407(1–2):184–9.
Wang Y, Wang C, Wang Y, Luo F, Yan X, Qian Z. Micelles of methoxy poly(ethylene glycol)-poly(epsilon-caprolactone) as a novel drug delivery vehicle for tacrolimus. J Biomed Nanotechnol. 2013;9(2):147–57.
Xu W, Ling P, Zhang T. Toward immunosuppressive effects on liver transplantation in rat model: tacrolimus loaded poly(ethylene glycol)-poly(D,L-lactide) nanoparticle with longer survival time. Int J Pharm. 2014;460(1–2):173–80.
Zhang D, Pan X, Wang S, Zhai Y, Guan J, Fu Q, et al. Multifunctional poly(methyl vinyl ether-co-maleic anhydride)-graft-hydroxypropyl-β-cyclodextrin amphiphilic copolymer as an oral high-performance delivery carrier of Tacrolimus. Mol Pharm. 2015;12(7):2337–51.
Shin TH, Ho MJ, Kim SR, Im SH, Kim CH, Lee S, et al. Formulation and in vivo pharmacokinetic evaluation of ethyl cellulose-coated sustained release multiple-unit system of tacrolimus. Int J Biol Macromol. 2018;109:544–50.
Wang M, Sun J, Zhai Y, Lian H, Luo C, Li L, et al. Enteric polymer based on pH-responsive aliphatic polycarbonate functionalized with vitamin E to facilitate oral delivery of tacrolimus. Biomacromolecules. 2015;16(4):1179–90.
Mohammed M, Mansell H, Shoker A, Wasan KM, Wasan EK. Development and in vitro characterization of chitosan-coated polymeric nanoparticles for oral delivery and sustained release of the immunosuppressant drug mycophenolate mofetil. Drug Dev Ind Pharm. 2019;45(1):76–87.
Hopf U, Ramadori G. Physiology and pathophysiology of the reticuloendothelial system of the liver (author’s transl). Leber Magen Darm. 1980;10(5):277–83.
Hirn S, Semmler-Behnke M, Schleh C, Wenk A, Lipka J, Schäffler M, et al. Particle size-dependent and surface charge-dependent biodistribution of gold nanoparticles after intravenous administration. Eur J Pharm Biopharm. 2011;77(3):407–16.
Poon W, Zhang YN, Ouyang B, Kingston BR, Wu JLY, Wilhelm S, et al. Elimination pathways of nanoparticles. ACS Nano. 2019;13(5):5785–98.
Stern ST, Adiseshaiah PP, Crist RM. Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Part Fibre Toxicol. 2012;9:20.
Longmire M, Choyke PL, Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine (Lond). 2008;3(5):703–17.
Sindhwani S, Syed AM, Ngai J, Kingston BR, Maiorino L, Rothschild J, et al. The entry of nanoparticles into solid tumours. Nat Mater. 2020;19(5):566–75.
Owen SC, Chan DPY, Shoichet MS. Polymeric micelle stability. Nano Today. 2012;7(1):53–65.
Fan W, Zhang L, Li Y, Wu H. Recent progress of crosslinking strategies for polymeric micelles with enhanced drug delivery in cancer therapy. Curr Med Chem. 2019;26(13):2356–76.
Lin M, Dai Y, Xia F, Zhang X. Advances in non-covalent crosslinked polymer micelles for biomedical applications. Mater Sci Eng C Mater Biol Appl. 2021;119:111626.
Xiao K, Li Y, Luo J, Lee JS, Xiao W, Gonik AM, et al. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials. 2011;32(13):3435–46.
Lipka J, Semmler-Behnke M, Sperling RA, Wenk A, Takenaka S, Schleh C, et al. Biodistribution of PEG-modified gold nanoparticles following intratracheal instillation and intravenous injection. Biomaterials. 2010;31(25):6574–81.
Srisa-Nga K, Mankhetkorn S, Okonogi S, Khonkarn R. Delivery of superparamagnetic polymeric Micelles loaded with quercetin to Hepatocellular Carcinoma cells. J Pharm Sci. 2019;108(2):996–1006.
Kamaly N, Yameen B, Wu J, Farokhzad OC. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of Controlling Drug Release. Chem Rev. 2016;116(4):2602–63.
Acknowledgements
We acknowledge the financial support by the National Natural Science Foundation of China (52173145, 81901627, 51803209, and U20A20360), the Jilin Provincial Science and Technology Development Program (20210101063JC), the Department of Finance of Jilin Province (JLSCZD2019-036), and the Youth Innovation Promotion Association of CAS (2022226).
Funding
This work was supported by the National Natural Science Foundation of China (52173145, 81901627, 51803209, and U20A20360), the Jilin Provincial Science and Technology Development Program (20210101063JC), the Department of Finance of Jilin Province (JLSCZD2019-036), and the Youth Innovation Promotion Association of CAS (2022226).
Author information
Authors and Affiliations
Contributions
GL, CX and PZ selected the topic and designed the review structure. FL wrote the manuscript. YY, ML and YC revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors consent to publication.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Luo, F., Yu, Y., Li, M. et al. Polymeric nanomedicines for the treatment of hepatic diseases. J Nanobiotechnol 20, 488 (2022). https://doi.org/10.1186/s12951-022-01708-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-022-01708-y







